


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 9-7-2017
Date: 21-2-2019
Date: 28-12-2016
|
Silicon does not double bond with oxygen. Because silicon atoms are larger than carbon atoms, silicon-oxygen bonds are longer than carbon-oxygen bonds. Consider a hypothetical silicon-oxygen double bond, analogous to the carbon-oxygen double bond discussed above. Because silicon-oxygen bonds are longer than carbon-oxygen equivalents, the p orbitals on silicon and oxygen cannot overlap enough to form a stable pi bond. Therefore, only single bonds are formed.
There are several structures for silicon dioxide. One of the simplest is shown below:
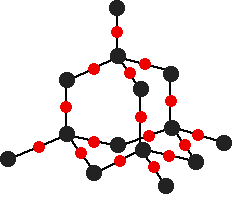
This is similar to the structure of diamond, with each of the silicon atoms bridged to its four neighbors via an oxygen atom, forming a large network covalent structure. Strong bonds in three dimensions make silicon dioxide a hard, high melting point solid.



|
|
|
|
لخفض ضغط الدم.. دراسة تحدد "تمارين مهمة"
|
|
|
|
|
|
|
طال انتظارها.. ميزة جديدة من "واتساب" تعزز الخصوصية
|
|
|
|
|
|
|
عوائل الشهداء: العتبة العباسية المقدسة سبّاقة في استذكار شهداء العراق عبر فعالياتها وأنشطتها المختلفة
|
|
|