


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 26-11-2018
Date: 26-10-2018
Date: 25-1-2019
|
We get most elements from nature in the form of minerals. In nature, phosphorus exists in the form of phosphates. Rocks containing phosphate are fluoroapatite (3Ca3(PO4)2⋅CaF2),chloroapaptite, (3Ca3(PO4)2⋅CaCl2), and hydroxyapatite (3Ca3(PO4)2⋅Ca(OH)2). These minerals are very similar to the bones and teeth. The arrangements of atoms and ions of bones and teeth are similar to those of the phosphate containing rocks. In fact, when the OH− ions of the teeth are replaced by F−, the teeth resist decay. This discovery led to a series of social and economical issues.
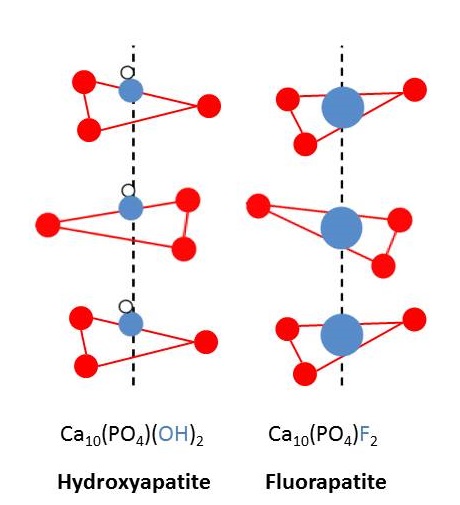
Figure 1: (left) Fluoride ions (F– ) replace hydroxyl groups (OH– ) in hydroxyapatite to form fluorapatite in the tooth enamel. (right) A portion of the apatite crystal lattice is depicted showing the replacement of hydroxide for fluoride (big blue circles). Image used with permission (Public Domain; Delmar Larsen).
Nitrogen, phosphorus and potassium are key ingredients for plants, and their contents are key in all forms of fertilizers. From an industrial and economical view point, phosphorus-containing compounds are important commodities. Thus, chemistry of phosphorus has academic, commercial and industrial interests.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|