


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 28-9-2018
Date: 28-9-2018
Date: 28-9-2018
|
REACTION RATE
The rate of a reaction tells as to what speed the reaction occurs. Let us consider a simple reaction
A → B
The concentration of the reactant A decreases and that of B increases as time passes. The rate of reactions is defined as the change in concentration of any of reactant or products per unit time. For the given reaction the rate of reaction may be equal to the rate of disappearance of A which is equal to the rate of appearance of B. Thus rate of reaction = rate of disappearance of A = rate of appearance of B
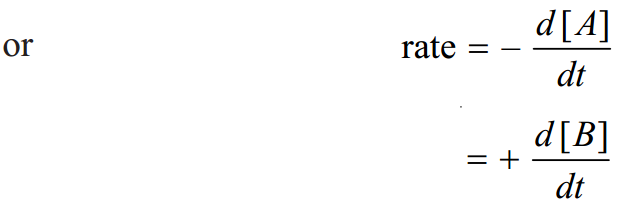
where [ ] represents the concentration in moles per liter whereas ‘d’ represents infinitesimally small change in concentration. Negative sign shows the concentration of the reactant A decreases whereas the positive sign indicates the increase in concentration of the product B.



|
|
|
|
دخلت غرفة فنسيت ماذا تريد من داخلها.. خبير يفسر الحالة
|
|
|
|
|
|
|
ثورة طبية.. ابتكار أصغر جهاز لتنظيم ضربات القلب في العالم
|
|
|
|
|
|
|
سماحة السيد الصافي يؤكد ضرورة تعريف المجتمعات بأهمية مبادئ أهل البيت (عليهم السلام) في إيجاد حلول للمشاكل الاجتماعية
|
|
|