

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Soaps and Detergents
المؤلف:
William Reusch
المصدر:
Virtual Textbook of Organic Chemistry
الجزء والصفحة:
............
17-8-2018
4506
Soaps and Detergents
Carboxylic acids and salts having alkyl chains longer than eight carbons exhibit unusual behavior in water due to the presence of both hydrophilic (CO2) and hydrophobic (alkyl) regions in the same molecule. Such molecules are termed amphiphilic (Gk. amphi = both) or amphipathic.
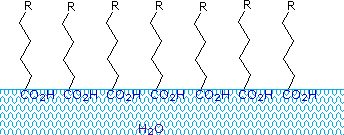
Fatty acids made up of ten or more carbon atoms are nearly insoluble in water, and because of their lower density, float on the surface when mixed with water. Unlike paraffin or other alkanes, which tend to puddle on the waters surface, these fatty acids spread evenly over an extended water surface, eventually forming a monomolecular layer in which the polar carboxyl groups are hydrogen bonded at the water interface, and the hydrocarbon chains are aligned together away from the water. This behavior is illustrated in the diagram on the right. Substances that accumulate at water surfaces and change the surface properties are called surfactants.
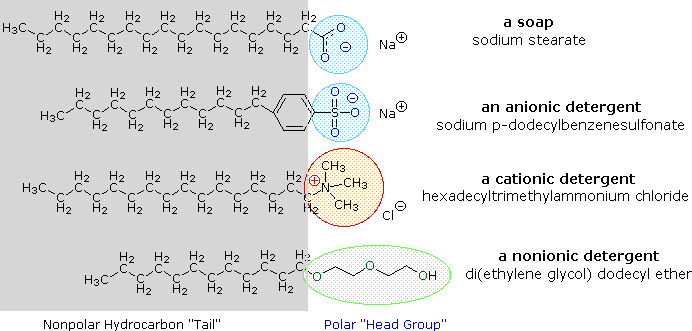
Alkali metal salts of fatty acids are more soluble in water than the acids themselves, and the amphiphilic character of these substances also make them strong surfactants. The most common examples of such compounds are soaps and detergents, four of which are shown below. Note that each of these molecules has a nonpolar hydrocarbon chain, the "tail", and a polar (often ionic) "head group". The use of such compounds as cleaning agents is facilitated by their surfactant character, which lowers the surface tension of water, allowing it to penetrate and wet a variety of materials.
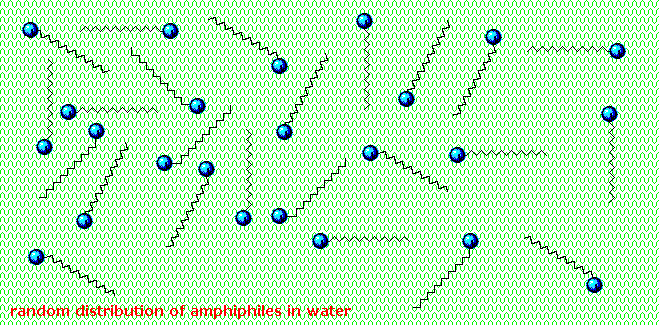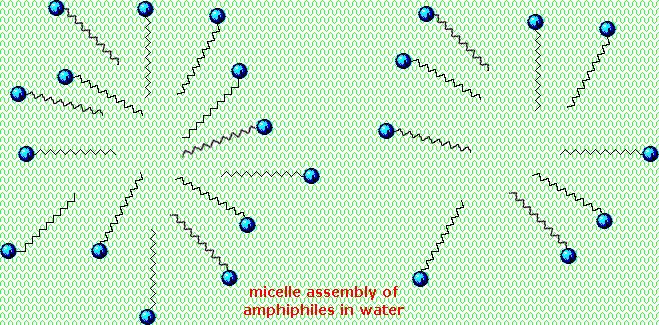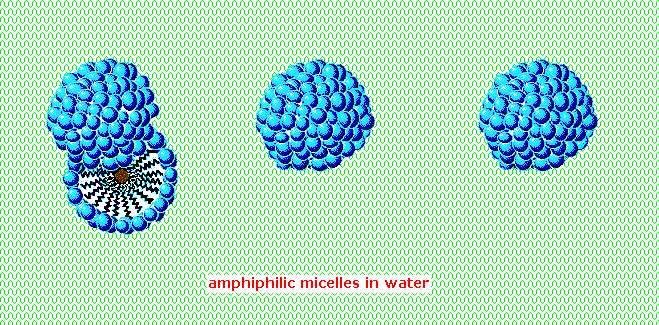
Very small amounts of these surfactants dissolve in water to give a random dispersion of solute molecules. However, when the concentration is increased an interesting change occurs. The surfactant molecules reversibly assemble into polymolecular aggregates called micelles.
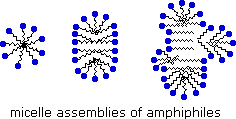
By gathering the hydrophobic chains together in the center of the micelle, disruption of the hydrogen bonded structure of liquid water is minimized, and the polar head groups extend into the surrounding water where they participate in hydrogen bonding. These micelles are often spherical in shape, but may also assume cylindrical and branched forms, as illustrated on the right. Here the polar head group is designated by a blue circle, and the nonpolar tail is a zig-zag black line.
An animated display of micelle formation is presented below. Notice the brownish material in the center of the three-dimensional drawing on the left. This illustrates a second important factor contributing to the use of these amphiphiles as cleaning agents. Micelles are able to encapsulate nonpolar substances such as grease within their hydrophobic center, and thus solubilize it so it is removed with the wash water. Since the micelles of anionic amphiphiles have a negatively charged surface, they repel one another and the nonpolar dirt is effectively emulsified. To summarize, the presence of a soap or a detergent in water facilitates the wetting of all parts of the object to be cleaned, and removes water-insoluble dirt by incorporation in micelles. If the animation has stopped, it may be restarted by clicking on it.
The oldest amphiphilic cleaning agent known to humans is soap. Soap is manufactured by the base-catalyzed hydrolysis (saponification) of animal fat (see below). Before sodium hydroxide was commercially available, a boiling solution of potassium carbonate leached from wood ashes was used. Soft potassium soaps were then converted to the harder sodium soaps by washing with salt solution. The importance of soap to human civilization is documented by history, but some problems associated with its use have been recognized. One of these is caused by the weak acidity (pKa ca. 4.9) of the fatty acids. Solutions of alkali metal soaps are slightly alkaline (pH 8 to 9) due to hydrolysis. If the pH of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a scum. A second problem is caused by the presence of calcium and magnesium salts in the water supply (hard water). These divalent cations cause aggregation of the micelles, which then deposit as a dirty scum.
These problems have been alleviated by the development of synthetic amphiphiles called detergents (or syndets). By using a much stronger acid for the polar head group, water solutions of the amphiphile are less sensitive to pH changes. Also the sulfonate functions used for virtually all anionic detergents confer greater solubility on micelles incorporating the alkaline earth cations found in hard water. Variations on the amphiphile theme have led to the development of other classes, such as the cationic and nonionic detergents shown above. Cationic detergents often exhibit germicidal properties, and their ability to change surface pH has made them useful as fabric softeners and hair conditioners. These versatile chemical "tools" have dramatically transformed the household and personal care cleaning product markets over the past fifty years.
 الاكثر قراءة في الدهون
الاكثر قراءة في الدهون
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















