

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Directed Aldol Reactions
المؤلف:
William Reusch
المصدر:
Virtual Textbook of Organic Chemistry
الجزء والصفحة:
............
5-8-2018
1909
Directed Aldol Reactions
The aldol reaction is potentially a powerful synthetic tool. As demonstrated by the following equation, a directed aldol reaction between different aldehydes would produce a single crossed aldol product (the new bond is colored maroon) and create two new stereogenic centers (colored blue). However, conducting this reaction with aqueous NaOH usually generates a mixture of four different adducts, and frequently β-elimination of water when possible.
R1CH2CHO |
+ |
R2CH2CHO |
base |
R1CH2CH(OH)—CH(R2)CHO |
| Carbonyl Acceptor | Enolate Donor | One Crossed Aldol Product |
In this section methods of controlling the enolate donor and carbonyl acceptor reactants to produce a single aldol product will be described and discussed. Several important principles must be taken into consideration for this purpose.
• The conventional aldol reaction is reversible. (aqueous base at room temperature)
• The initial aldol product may undergo beta-elimination of water, depending on reaction conditions.
• Regioselective enolate anion formation may be achieved by using differences in kinetic vs. equilibrium acidities of α-hydrogens.
• Enolate anions may be trapped as silyl ethers and used subsequently for controlled aldol reactions.
• Enolate-like species may be used for aldol-like reactions with carbonyl compounds, and hydrolyzed later to aldol products.
• Metal cations or other electrophilic moieties may act to orient the reactants in specific ways.
The reversibility of conventional aldol reactions is illustrated by the crossed aldol condensation of 2-butanone with benzaldehyde, illustrated below. The methyl hydrogens are kinetically more acidic than the methylene hydrogens, so the α'-enolate is generated more rapidly than the more substituted α-enolate. As a rule, ketone enolate bases react more rapidly with aldehydes than with ketones, so self condensation of the ketone is minimal. Both enolate anions add reversibly to benzaldehyde to form β-hydroxy ketones; however, the α-enolate product (on the left) reverts to reactants faster than it eliminates water. In contrast, the isomeric α'-enolate product (on the right) undergoes rapid β-elimination to the observed 5-phenyl-4-penten-3-one final product.
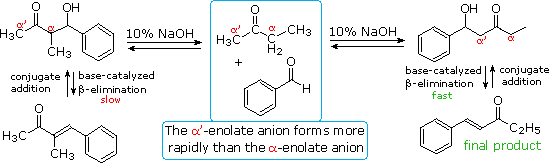
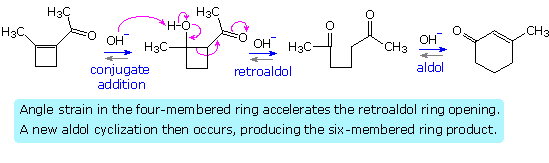
Acid catalysis of the 2-butanone-benzaldehyde reaction gives the isomer, 3-methyl-4-phenyl-3-buten-2-one, as the chief product, thanks to the predominance of the more substituted α-enol as an intermediate. In this case both β-hydroxy ketone intermediates undergo reversible dehydration.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















