


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 1-8-2018
Date: 2-9-2019
Date: 20-9-2020
|
The two most commonly used compounds of this kind are alkyl lithium reagents and Grignard reagents. They are prepared from alkyl and aryl halides, as discussed earlier. These reagents are powerful nucleophiles and very strong bases (pKa's of saturated hydrocarbons range from 42 to 50), so they bond readily to carbonyl carbon atoms, giving alkoxide salts of lithium or magnesium. Because of their ring strain, epoxides undergo many carbonyl-like reactions, as noted previously. Reactions of this kind are among the most important synthetic methods available to chemists, because they permit simple starting compounds to be joined to form more complex structures. Examples are shown in the following diagram.
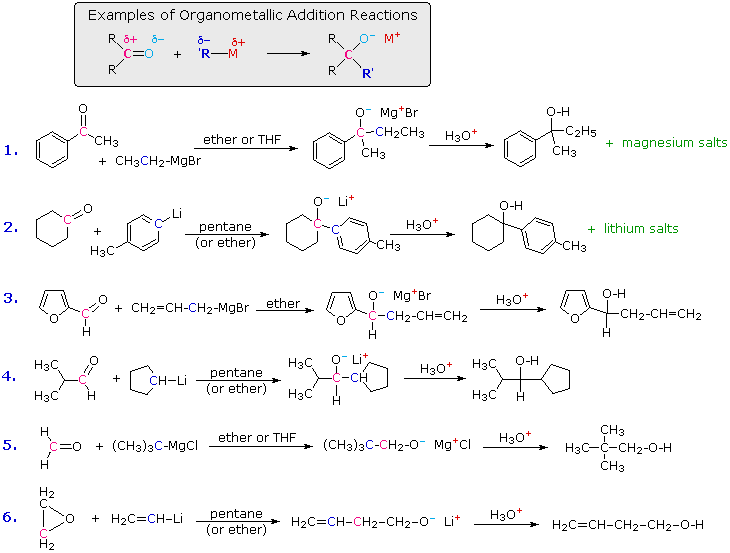
A common pattern, shown in the shaded box at the top, is observed in all these reactions. The organometallic reagent is a source of a nucleophilic alkyl or aryl group (colored blue), which bonds to the electrophilic carbon of the carbonyl group (colored magenta). The product of this addition is a metal alkoxide salt, and the alcohol product is generated by weak acid hydrolysis of the salt. The first two examples show that water soluble magnesium or lithium salts are also formed in the hydrolysis, but these are seldom listed among the products, as in the last four reactions. Ketones react with organometallic reagents to give 3º-alcohols; most aldehydes react to produce 2º-alcohols; and formaldehyde and ethylene oxide react to form 1º-alcohols (examples #5 & 6). When a chiral center is formed from achiral reactants (examples #1, 3 & 4) the product is always a racemic mixture of enantiomers.
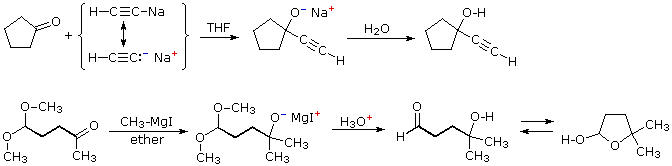
Two additional examples of the addition of organometallic reagents to carbonyl compounds are informative. The first demonstrates that active metal derivatives of terminal alkynes function in the same fashion as alkyl lithium and Grignard reagents. The second example again illustrates the use of acetal protective groups in reactions with powerful nucleophiles. Following acid-catalyzed hydrolysis of the acetal, the resulting 4-hydroxyaldehyde is in equilibrium with its cyclic hemiacetal.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|