

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Acetal Formation
المؤلف:
William Reusch
المصدر:
Virtual Textbook of Organic Chemistry
الجزء والصفحة:
............
1-8-2018
3118
Acetal Formation
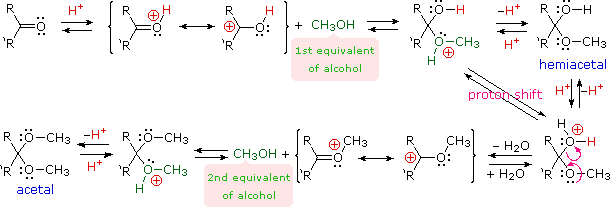
Acetals are geminal-diether derivatives of aldehydes or ketones, formed by reaction with two equivalents of an alcohol and elimination of water. Ketone derivatives of this kind were once called ketals, but modern usage has dropped that term. The following equation shows the overall stoichiometric change in acetal formation, but a dashed arrow is used because this conversion does not occur on simple mixing of the reactants.
R2C=O + 2 R'OH  R2C(OR')2 + H2O (an acetal)
R2C(OR')2 + H2O (an acetal)
In order to achieve effective acetal formation two additional features must be implemented. First, an acid catalyst must be used; and second, the water produced with the acetal must be removed from the reaction. The latter is important, since acetal formation is reversible. Indeed, once pure acetals are obtained they may be hydrolyzed back to their starting components by treatment with aqueous acid.
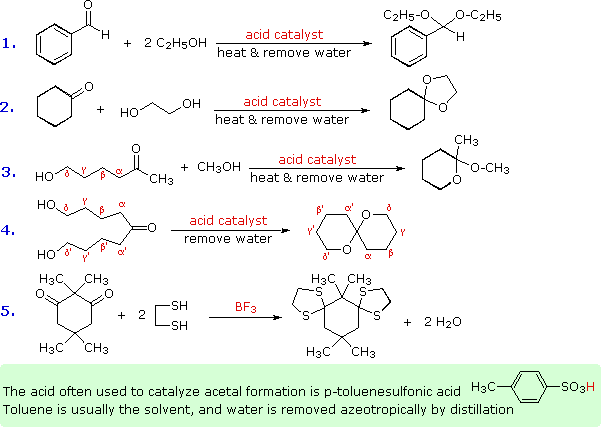
Some examples of acetal formation are presented in the following diagram. As noted, p-toluenesulfonic acid (pKa = -2) is often the catalyst for such reactions. Two equivalents of the alcohol reactant are needed, but these may be provided by one equivalent of a diol (example #2). Intramolecular involvement of a gamma or delta hydroxyl group (as in examples #3 and 4) may occur, and is often more facile than the intermolecular reaction. Thiols (sulfur analogs of alcohols) give thioacetals (example #5). In this case the carbonyl functions are relatively hindered, but by using excess ethanedithiol as the solvent and the Lewis acid BF3 as catalyst a good yield of the bis-thioacetal is obtained. Thioacetals are generally more difficult to hydrolyze than are acetals.
The importance of acetals as carbonyl derivatives lies chiefly in their stability and lack of reactivity in neutral to strongly basic environments. As long as they are not treated by acids, especially aqueous acid, acetals exhibit all the lack of reactivity associated with ethers in general.
Among the most useful and characteristic reactions of aldehydes and ketones is their reactivity toward strongly nucleophilic (and basic) metallo-hydride, alkyl and aryl reagents (to be discussed shortly). If the carbonyl functional group is converted to an acetal these powerful reagents have no effect; thus, acetals are excellent protective groups, when these irreversible addition reactions must be prevented.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















