


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 29-8-2019
Date: 31-10-2019
Date: 10-10-2019
|
Hydrogen Bonding
The presence of a hydrogen bond donor near a carboxyl group may act to enhance its acidity, as demonstrated by the three isomeric hydroxybenzoic acids and three isomeric benzenedicarboxylic acids (phthalic acids) shown in the following table. Compared with benzoic acid, the meta and para-isomers display expected changes in acidity, due to combined inductive and resonance effects. However, the ortho isomers are both roughly 15 times more acidic, even though the hydroxyl and carboxyl substituents have opposite influences in the para-location.
|
ortho relationship |
pKa1 |
pKa2 |
meta relationship |
pKa1 |
pKa2 |
para relationship |
pKa1 |
pKa2 |
||
|
salicylic acid |
2.97 |
13.44 |
meta-hydroxybenzoic acid |
4.08 |
9.91 |
para-hydroxybenzoic acid |
4.58 |
9.40 |
||
|
phthalic acid |
2.98 |
5.28 |
isophthalic acid |
3.46 |
4.46 |
terephthalic acid |
3.51 |
4.82 |
Intramolecular hydrogen bonding of an ortho OH donor to the carbonyl oxygen of the carboxyl group, acting as an acceptor, increases the positive charge on the carbonyl carbon and consequently the acidity of the carboxyl OH. This is illustrated for salicylic acid in the following diagram. Phthalic acid engages in a similar seven-membered cyclic hydrogen bond with a similar outcome. This intramolecular hydrogen bonding also explains the decreased acidity of the remaining acidic function - that is the phenolic OH in salicylic acid and the second carboxyl group in phthalic acid.
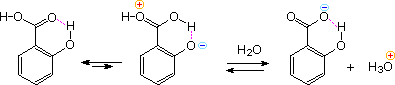
The stereoisomeric 2-butenedioic acids, maleic and fumaric acid display a similar behavior. The cis isomer, maleic acid, has pKa1 = 2.00 and pKa2 = 6.50. This contrasts with the values for the trans-isomer, fumaric acid, pKa1 = 3.00 and pKa2 = 4.50.



|
|
|
|
دخلت غرفة فنسيت ماذا تريد من داخلها.. خبير يفسر الحالة
|
|
|
|
|
|
|
ثورة طبية.. ابتكار أصغر جهاز لتنظيم ضربات القلب في العالم
|
|
|
|
|
|
|
العتبة العباسية المقدسة تستعد لإطلاق الحفل المركزي لتخرج طلبة الجامعات العراقية
|
|
|