
Mean Free Path
 المؤلف:
Professor John W. Norbury
المؤلف:
Professor John W. Norbury
 المصدر:
ELEMENTARY MECHANICS & THERMODYNAMICS
المصدر:
ELEMENTARY MECHANICS & THERMODYNAMICS
 الجزء والصفحة:
p 232
الجزء والصفحة:
p 232
 1-1-2017
1-1-2017
 2114
2114
Mean Free Path
Even though room temperature air molecules have a large RMS speed vRMS 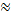 500 m/sec, that does not mean that they move across a room in a fraction of a second. If you open a bottle of perfume at one end of a room, it takes a while for you to notice the smell at the other end of the room. This is because the molecules undergo an enormous number of collisions on their way across the room. The mean free path λ is the average distance that a molecule travels in between collisions. It is given by
500 m/sec, that does not mean that they move across a room in a fraction of a second. If you open a bottle of perfume at one end of a room, it takes a while for you to notice the smell at the other end of the room. This is because the molecules undergo an enormous number of collisions on their way across the room. The mean free path λ is the average distance that a molecule travels in between collisions. It is given by
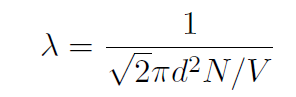
where d is the average diameter of a molecule, and N/V is the average number of molecules per unit volume.
 الاكثر قراءة في الفيزياء الكيميائية
الاكثر قراءة في الفيزياء الكيميائية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


