
The temperature-dependence of reaction enthalpies
 المؤلف:
Peter Atkins، Julio de Paula
المؤلف:
Peter Atkins، Julio de Paula
 المصدر:
ATKINS PHYSICAL CHEMISTRY
المصدر:
ATKINS PHYSICAL CHEMISTRY
 الجزء والصفحة:
ص56-57
الجزء والصفحة:
ص56-57
 2025-11-03
2025-11-03
 35
35
The temperature-dependence of reaction enthalpies
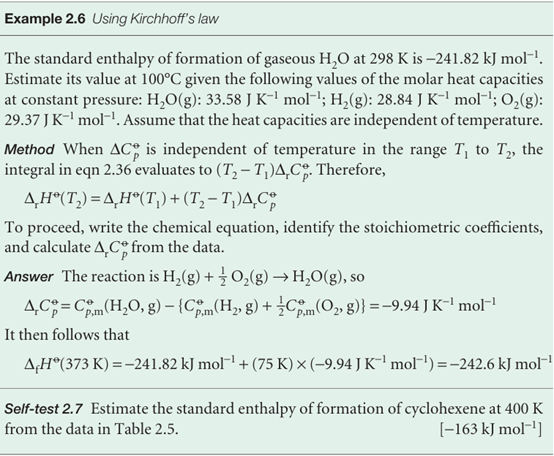
The standard enthalpies of many important reactions have been measured at different temperatures. However, in the absence of this information, standard reaction enthalpies at different temperatures may be calculated from heat capacities and the reaction enthalpy at some other temperature (Fig. 2.19). In many cases heat capacity data are more accurate that reaction enthalpies so, providing the information is avail-able, the procedure we are about to describe is more accurate that a direct measure ment of a reaction enthalpy at an elevated temperature. It follows from eqn 2.23a that, when a substance is heated from T1 to T2, its enthalpy changes from H(T1) to
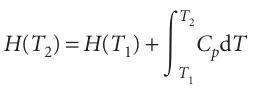
(We have assumed that no phase transition takes place in the temperature range of interest.) Because this equation applies to each substance in the reaction, the standard reaction enthalpy changes from ∆rHo(T1) to
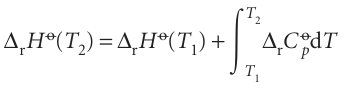
where ∆rCpo is the difference of the molar heat capacities of products and reactants under standard conditions weighted by the stoichiometric coefficients that appear in the chemical equation:
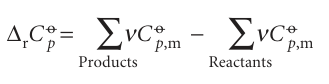
Equation 2.36 is known as Kirchhoff’s law. It is normally a good approximation to assume that ∆rCp is independent of the temperature, at least over reasonably limited ranges, as illustrated in the following example. Although the individual heat capacities may vary, their difference varies less significantly. In some cases the temperature dependence of heat capacities is taken into account by using eqn 2.25.
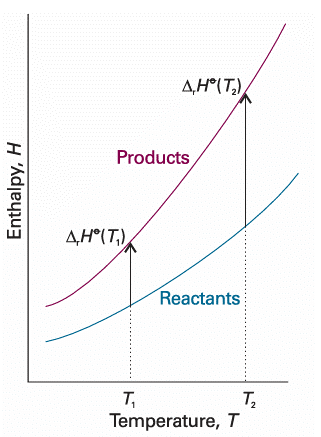
Fig. 2.19 An illustration of the content of Kirchhoff’s law. When the temperature is increased, the enthalpy of the products and the reactants both increase, but may do so to different extents. In each case, the change in enthalpy depends on the heat capacities of the substances. The change in reaction enthalpy reflects the difference in the changes of the enthalpies.
 الاكثر قراءة في مواضيع عامة في الكيمياء الفيزيائية
الاكثر قراءة في مواضيع عامة في الكيمياء الفيزيائية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


