
Enthalpies of chemical change
 المؤلف:
Peter Atkins، Julio de Paula
المؤلف:
Peter Atkins، Julio de Paula
 المصدر:
ATKINS PHYSICAL CHEMISTRY
المصدر:
ATKINS PHYSICAL CHEMISTRY
 الجزء والصفحة:
ص51-53
الجزء والصفحة:
ص51-53
 2025-11-03
2025-11-03
 41
41
Enthalpies of chemical change
Now we consider enthalpy changes that accompany chemical reactions. There are two ways of reporting the change in enthalpy that accompanies a chemical reaction. One is to write the thermochemical equation, a combination of a chemical equation and the corresponding change in standard enthalpy:
CH4(g) +2O2(g) →CO2(g)+2 H2O(l) ∆Ho=−890 KJ
Pure, separate reactants in their standard states →pure, separate products in their standard states , Except in the case of ionic reactions in solution, the enthalpy changes accompanying mixing and separation are insignificant in comparison with the contribution from the reaction itself. For the combustion of methane, the standard value refers to the reaction in which 1 mol CH4 in the form of pure methane gas at 1 bar reacts completely with 2 mol O2 in the form of pure oxygen gas to produce 1 mol CO2 as pure carbon dioxide at 1 bar and 2 mol H2O as pure liquid water at 1 bar; the numerical value is for the reaction at 298 K.
Alternatively, we write the chemical equation and then report the standard reaction enthalpy, ∆rHo. Thus, for the combustion of reaction, we write
CH4(g) +2O2(g) →CO2(g)+2 H2O(l) ∆rHO=−890 kJ mol−1
For the reaction
2 A + B →3 C +D
the standard reaction enthalpy is ∆rHo= {3Ho m(C)+Hom(D)} −{2Hom(A) +Hom(B)}
Note how the ‘per mole’ of ∆rHo comes directly from the fact that molar enthalpies appear in this expression. We interpret the ‘per mole’ by noting the stoichiometic coefficients in the chemical equation. In this case ‘per mole’ in ∆rHo means ‘per 2 mol A’, ‘per mole B’, ‘per 3 mol C’, or ‘per mol D’. In general,
∆rHo= Products ∑νHom − Reactants∑νHom
where in each case the molar enthalpies of the species are multiplied by their stoichio metric coefficients, ν.6 Some standard reaction enthalpies have special names and a particular significance. For instance, the standard enthalpy of combustion, ∆cHo, is the standard reaction enthalpy for the complete oxidation of an organic compound to CO2 gas and liquid H2O if the compound contains C, H, and O, and to N2 gas if N is also present. An example is the combustion of glucose:
C6H12O6(s) + 6O2(g) → 6CO2(g) + 6H2O(l) ∆cHo=−2808 kJ mol−1
The value quoted shows that 2808 kJ of heat is released when 1 mol C6H12O6 burns under standard conditions (at 298 K). Some further values are listed in Table 2.5.
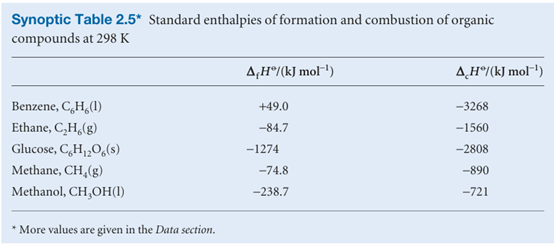
The thermochemical properties of fuels Table 2.6 and foods are commonly discussed in terms of their specific enthalpy, the enthalpy of combustion per gram of material. Thus, if the standard enthalpy of combustion is ∆cHo and the molar mass of the com pound is M, then the specific enthalpy is ∆cHo/M. Table 2.6 lists the specific enthalpies of several fuels. A typical 18–20-year-old man requires a daily input of about 12 MJ; a woman of the same age needs about 9 MJ. If the entire consumption were in the form of glucose (3; which has a specific enthalpy of 16 kJ g−1), that would require the consumption of 750 g of glucose for a man and 560 g for a woman. In fact, digestible carbohydrates have a slightly higher specific enthalpy (17 kJ g−1) than glucose itself, so a carbohydrate
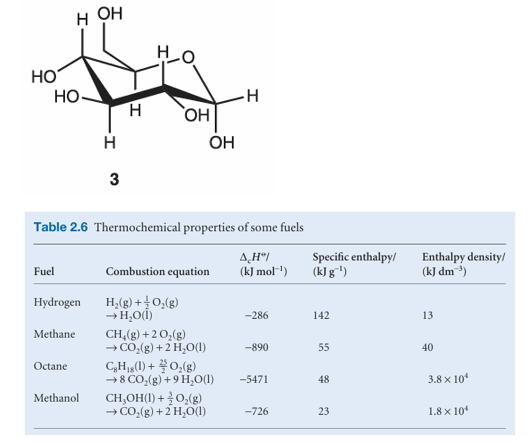
diet is slightly less daunting than a pure glucose diet, as well as being more appropriate in the form of fibre, the indigestible cellulose that helps move digestion products through the intestine. The specific enthalpy of fats, which are long-chain esters like tristearin (beef fat), is much greater than that of carbohydrates, at around 38 kJ g−1, slightly less than the value for the hydrocarbon oils used as fuel (48 kJ g−1). Fats are commonly used as an energy store, to be used only when the more readily accessible carbohydrates have fallen into short supply. In Arctic species, the stored fat also acts as a layer of insulation; in desert species (such as the camel), the fat is also a source of water, one of its oxidation products. Proteins are also used as a source of energy, but their components, the amino acids, are often too valuable to squander in this way, and are used to construct other proteins instead. When proteins are oxidized (to urea, CO(NH2)2), the equivalent enthalpy density is comparable to that of carbohydrates. The heat released by the oxidation of foods needs to be discarded in order to maintain body temperature within its typical range of 35.6–37.8°C. A variety of mechanisms contribute to this aspect of homeostasis, the ability of an organism to counteract environmental changes with physiological responses. The general uniformity of temperature throughout the body is maintained largely by the flow of blood. When heat needs to be dissipated rapidly, warm blood is allowed to flow through the capillaries of the skin, so producing flushing. Radiation is one means of discarding heat; another is evaporation and the energy demands of the enthalpy of vaporization of water. Evaporation removes about 2.4 kJ per gram of water perspired. When vigorous exercise promotes sweating (through the influence of heat selectors on the hypothalamus), 1–2 dm3 of perspired water can be produced per hour, corresponding to a heat loss of 2.4–5.0 MJ h−1.
 الاكثر قراءة في مواضيع عامة في الكيمياء الفيزيائية
الاكثر قراءة في مواضيع عامة في الكيمياء الفيزيائية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


