

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Physical properties
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
الجزء والصفحة:
ص450-453
2025-09-23
139
Physical properties
Key points: The properties of the d metals are largely derived from their electronic structure, with the strength of metallic bonding peaking at Group 6; the lanthanide contraction is responsible for some of the anomalous behaviour of the metals in the 5d series. The d block of the periodic table contains the metals most important to modern society. It contains the immensely strong and light titanium, the major components of most steels (Fe, Cr, Mn, Mo), the highly electrically conducting copper, the malleable gold and platinum, and the very dense osmium and iridium. To a large extent these properties derive from the nature of the metallic bonding that binds the atoms together.
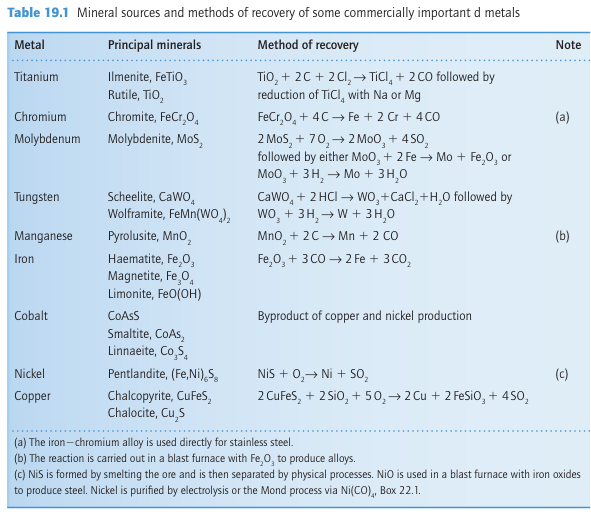

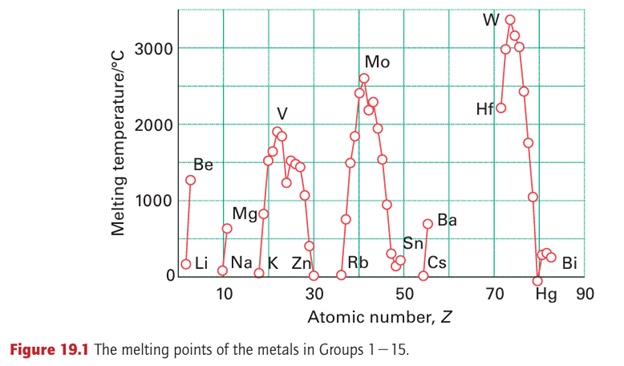
Metallic bonding was covered in Chapter 3, which introduced the concept of band structure. Generally speaking, the same band structure is present for all the d-block metals and arises from the overlap of the (n+1)s orbitals to give an s band and of the nd orbitals to give a d band. The principal differences between the metals is the number of electrons available to occupy these bands: Ti (3d24s2) has four bonding electrons, V (3d34s2) five, Cr (3d54s1) six, and so on. The lower, net bonding region of the valence band is therefore progressively filled with electrons on going to the right across the block, which results in stronger bonding, until around Group 7 (at Mn, Tc, Re) when the electrons begin to populate the upper, net antibonding part of the band. This trend in bonding strength is reflected in the increase in melting point from the low-melting alkali metals (effectively only one bonding electron for each atom, resulting in melting points typically less than 100ºC) up to Cr, and its decline thereafter to the low-melting Group 12 metals (mercury being a liquid at room temperature, Fig. 19.1 and Section 9.2). The strength of metallic bonding in tungsten is such that its melting point (3410ºC) is exceeded by only one other element, carbon. The radii of d-metal ions depend on the effective charge of the nucleus, and ionic radii generally decrease on moving to the right as the atomic number increases. The radius of the metal atoms in the solid element is determined by a combination of the strength of the metallic bonding and the size of the ions. Thus, the separations of the centres of the atoms in the solid generally follow a similar pattern to the melting points: they decrease to the middle of the d block, followed by an increase back up to Group 12, with the smallest separations occurring in and near Groups 7 and 8. The atomic radii of the elements in the 5d series (Hf, Ta, W,...) are not much bigger than those of their 4d-series congeners (Zr, Nb, Mo,...). In fact, the atomic radius of Hf
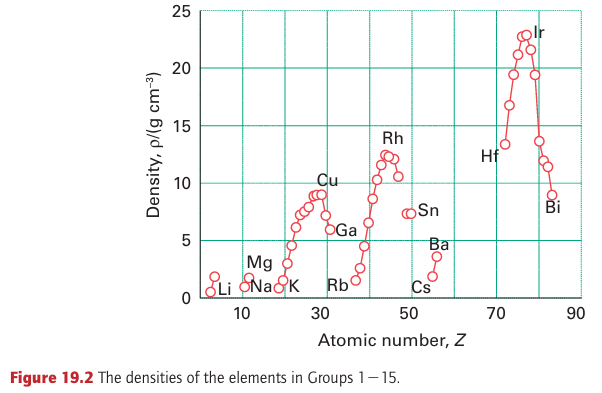
is smaller than that of Zr even though it appears in a later period. To understand this anomaly, we need to consider the effect of the lanthanoids (the first row of the f block). The intervention of the lanthanoid elements in Period 6 corresponds to the occupation of the poorly shielding 4f orbitals. Because the atomic number has increased by 32 between Zr in Period 5 and its congener Hf in Period 6 without a corresponding increase in shielding, the overall effect is that the atomic radii of the 5d-series elements are much smaller than expected. This reduction in radius is the lanthanide contraction introduced in Section 1.9a. The lanthanide contraction also affects the ionization energies of the 5d-series elements, making them higher than expected on the basis of a straightforward extrapolation. Some of the metals—specifically Au, Pt, Ir, and Os—have such high ionization energies that they are unreactive under normal conditions. Atomic mass increases with atomic number, and the combination of this increase with the changes in the radii of the metal atoms in the metal lattice means that the mass densities of the elements reach a peak with Ir (density 22.65 g cm3). Figure 19.2 illustrates this trend.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















