

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Metal–organic polyhedra and frameworks
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
الجزء والصفحة:
ص676-678
2025-10-15
63
Metal–organic polyhedra and frameworks
Key points: Metal–organic polyhedra and frameworks offer tunable catalytic surfaces and high surface areas; they are assembled using supramolecular chemistry. A second class of three-dimensionally controlled nanostructures is metal–organic frameworks (MOFs). These frameworks are self-assembled from a careful choice of metal ions, bridging organic ligands, and/or metal–organic polyhedra (MOP). They offer an alternative approach to the synthesis of open porous materials using supramolecular assembly and are being called the ‘new zeolites’. These macroscopic materials have some of the highest reported surface areas and, as such, have found practical application for methane and hydrogen storage, catalysis, and drug delivery. As discussed earlier, supramolecular chemistry relies on bridging together nanobuilding blocks (NBBs) to assemble macroscopic materials. One new strategy in design is the incorporation of nanoporosity (pores with dimensions of 1 to 100 nm) within the NBB or MOP. The synthesis of a nanoporous cuboctahedron NBB (Fig. 25.30a) was achieved by linking rigid square molecules of Cu2(CO2)4 with m-BDC (1,3-benzenedicarboxylate). The overall NBB formula is Cu25(m-BDC)24(DMF)14(H2O)10(H2O)50(DMF)6 (C2H5OH)6 and is termed more conveniently MOP-1. This compound is constructed from 12 paddle-wheel units (themselves NBBs) bridged by m-BDC to yield a large metal-carboxylate polyhedron. The simplest way to view the overall structure is by considering its relationship to the cuboctahedron (shown in Fig. 25.13), where each square and link has been replaced by the paddle-wheel NBB and the m-BDC (two-connector) units, respectively, to give an expanded-augmented cuboctahedron (or a truncated cuboctahedron). The ability to synthsize the cuboctahedron illustrates the feasibility of obtaining crystals of large porous metal–organic polyhedra in which rigid NBBs are an integral part of a well-defined structure. More recently, a zeolitic imidazolate framework compound, ZIF-69, has been reported that can store 83 dm3 of carbon dioxide perlitre (1 dm3) of material at 0°C and 1 atm. MOPs have also been designed for selective catalysis that mimic active sites in enzymes. Enzymatic catalysis often relies on the highly constrained environment of substrates within the active site. Recent examples in supramolecular organometallic chemistry mimic this approach by using host–guest interactions. A supramolecular M4L6 tetrahedral assembly has been developed to provide a host that mediates a variety of organic and organometallic transformations, including the isomerization of allylic alcohols. The M4L612- host is formed from the self-assembly of four octahedral metal centres (M Fe (III) or Ga(III)) at the vertices and six bis-catecholamide naphthalene ligands (L4-) that span the edges of a tetrahedron (Fig. 25.30b). The host has been found to encapsulate monocationic guests
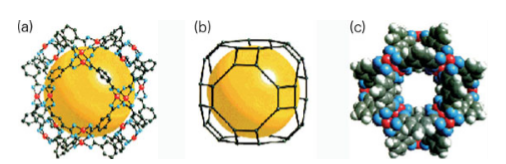
Figure 25.30a The crystal structure of MOP-1 (drawn using coordinates obtained for c-MOP-1) showing (a) 12 paddle-wheel units (Cu, red; O, blue; C, grey) linked by m-BDC to form (b) a large truncated cuboctahedron of 1.5 nm diameter void (yellow sphere); the grey spheres (carbon atoms) represent the polyhedron constructed by linking together only the carboxylate C atoms in (a) to form linked square NBBs, an arrangement that provides for (c) a large porous polyhedron with triangular and square windows. Hydrogen atoms in light grey; otherwise, same colouring scheme as in (a). (M. Eddaoudi, et al., J. Am. Chem. Soc., 2001, 123, 4368.) (Reproduced with permission from the American Chemical Society.)
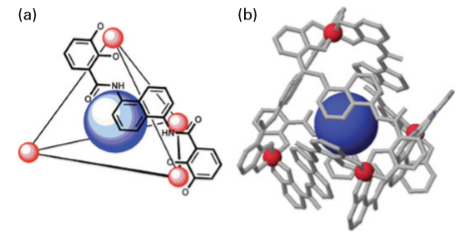
Figure 25.30b (a) Schematic of MOP structure of an M4L6 assembly with one of the edge-spanning ligands displayed containing an organometallic guest. (b) Crystal structure of Fe4L6 assembly with an organometallic guest. (D. H. Leung, et al., J. Am. Chem. Soc., 2007, 129, 2746.) (Reproduced with permission from the American Chemical Society.) ranging from NH4+ cations to cationic organometallic species containing Rh metal centres. The catalytic activity is controlled by the size and shape of the host cavity. Some of the key challenges in the area of mesostructured materials are achieving control over pore size and shape, the presence of counterions and solvents within the channels, the interpenetration of different networks, the framework structural instability in the absence of guest molecules, and the often-low thermal stability of the host framework. In some cases, the incorporation of solvent molecules can be used to achieve desired structures. For example, a robust three-dimensional porous structure of formula [Ln2(PDC)3(DMF)2]∞ has been constructed from lanthanoid (Ln) cations (Er3+ or Y3+) and the non-linear anionic bridging ligand, pyridine-3,5-dicarboxylate (PDC2–) in dimethylformamide (DMF). The solvated framework polymers undergo a solid-state, crystal-to-crystal reaction on heating. Through loss of both sorbed and coordinated solvent and rearrangement of the framework core a porous MOF structure is formed with retention of structural integrity (Fig. 25.31). These mesoporous materials have proven to be effective absorbants for H2, N2, and benzene.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















