

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Propylene oxide
المؤلف:
sami matar & Lewis. F. Hatch
المصدر:
Chemistry of PETROCHEMICAL PROCESSES
الجزء والصفحة:
p 221
31-8-2017
5852
Propylene oxide
Propylene oxide is similar in its structure to ethylene oxide, but due to the presence of an additional methyl group, it has different physical and chemical properties. It is a liquid that boils at 33.9°C, and it is only slightly soluble in water. (Ethylene oxide, a gas, is very soluble in water).
The main method to obtain propylene oxide is chlorohydrination followed by epoxidation. This older method still holds a dominant role in propylene oxide production. Chlorohydrination is the reaction between an olefin and hypochlorous acid. When propylene is the reactant, propylene chlorohydrin is produced. The reaction occurs at approximately 35°C and normal pressure without any catalyst:
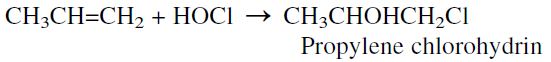
Approximately 87–90% yield could be achieved. The main by-product is propylene dichloride (6–9%). The next step is the dehydrochlorination of the chlorohydrin with a 5% Ca(OH)2 solution:
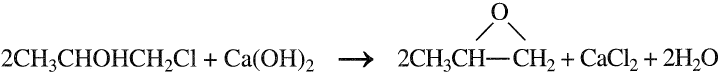
Propylene oxide is purified by steam stripping and then distillation. Byproduct propylene dichloride may be purified for use as a solvent or as a feed to the perchloroethylene process. The main disadvantage of the chlorohydrination process is the waste disposal of CaCl2. Figure 1.1 is a flow diagram of a typical chlorohydrin process. The second important process for propylene oxide is epoxidation with peroxides. Many hydroperoxides have been used as oxygen carriers for this reaction. Examples are t-butylhydroperoxide, ethylbenzene hydroperoxide, and peracetic acid. An important advantage of the process is that the coproducts from epoxidation have appreciable economic values. Epoxidation of propylene with ethylbenzene hydroperoxide is carried out at approximately 130°C and 35 atmospheres in presence of molybdenum catalyst. A conversion of 98% on the hydroperoxide has been reported:
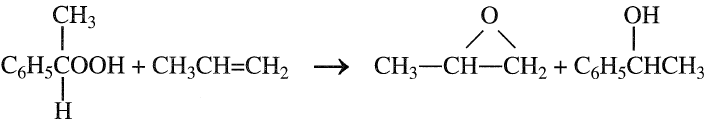
The coproduct α-phenylethyl alcohol could be dehydrated to styrene. Ethylbenzene hydroperoxide is produced by the uncatalyzed reaction of ethylbenzene with oxygen:
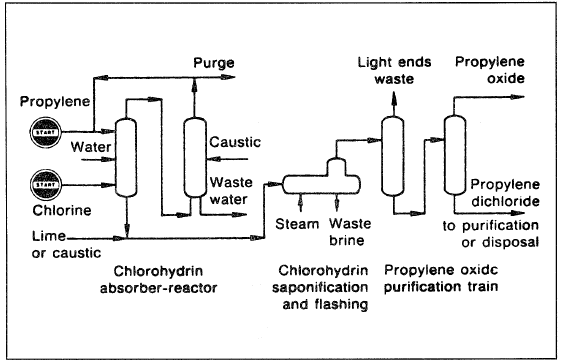
Figure 1.1. A flow diagram of a typical chlorohydrin process for producing propylene oxide.
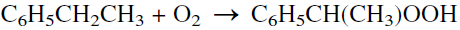
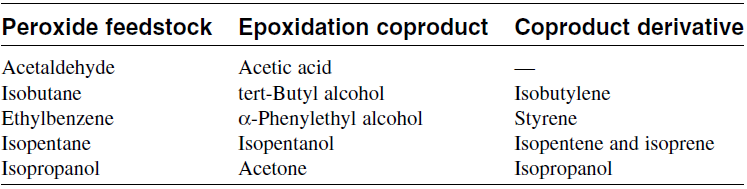
Table 1.1 shows those peroxides normally used for epoxidation of propylene and the coproducts with economic value.
Epoxidation with hydrogen peroxide has also been tried. The epoxidation reaction is catalyzed with compounds of As, Mo, and B, which are claimed to produce propylene oxide in high yield:

Table 1.1: Peroxides actually or potentially used to epoxidize propylene
 الاكثر قراءة في البترو كيمياويات
الاكثر قراءة في البترو كيمياويات
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















