
HYDRATION OF ISOBUTYLENE (Ter-Butyl Alcohol [(CH3)3COH])
 المؤلف:
sami matar & Lewis. F. Hatch
المؤلف:
sami matar & Lewis. F. Hatch
 المصدر:
Chemistry of PETROCHEMICAL PROCESSES
المصدر:
Chemistry of PETROCHEMICAL PROCESSES
 الجزء والصفحة:
p 253
الجزء والصفحة:
p 253
 5-9-2017
5-9-2017
 1852
1852
HYDRATION OF ISOBUTYLENE (Ter-Butyl Alcohol [(CH3)3COH])
The acid-catalyzed hydration of isobutylene produces ter-butyl alcohol. The reaction occurs in the liquid phase in the presence of 50–65% H2SO4 at mild temperatures (10–30°C). The yield is approximately 95%:

ter-Butyl alcohol (TBA) is used as a chemical intermediate because a tertiary butyl carbocation forms easily. It is also used as a solvent in pharmaceutical formulations, a paint remover, and a high-octane gasoline additive. The alcohol is a major by-product from the synthesis of propylene oxide using tertiary butyl hydroperoxide. Surplus ter-butyl alcohol could be used to synthesize highly pure isobutylene for MTBE production by a dehydration step. The reaction conditions, the catalyst used in a pilot-scale unit, and the yield are reviewed by Abraham and Prescott. It was concluded that MTBE conversion increases from 8 wt% to 88 wt% as the temperature increases from 400°F to 600°F at about 40 LHSV (liquid hourly space velocity). At a lower space velocity
(≈20 LHSV), conversion increased from 12 wt% to 99 wt% for the same temperature range. Figure 1.1 shows the effect of temperature and LHSV on the conversion:


Figure 1.1. Effect of temperature and liquid hourly space velocity on conversion.
Figure 1.2 is a simplified flow diagram of a TBA dehydration pilot unit.
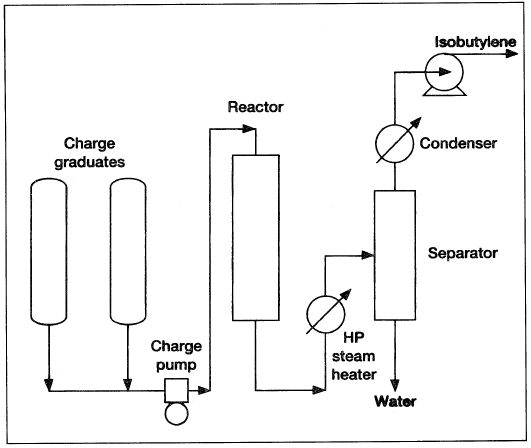
Figure 1.2. A simplified flow diagram of a tertiary butyl alcohol pilot plant.
 الاكثر قراءة في البترو كيمياويات
الاكثر قراءة في البترو كيمياويات
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


