

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Galvanic Cells and Standard Half-Cell Potentials
المؤلف:
Jerome L. Rosenberg and Lawrence M. Epstein
المصدر:
College Chemistry
الجزء والصفحة:
p 135
18-7-2017
1129
Galvanic Cells and Standard Half-Cell Potentials
Here we investigate some of the properties of galvanic cells, cells used to produce an electric potential. Luigi Galvani discovered the first such cell by accident in 1791. Following Galvani's discovery, Alessandro Volta developed a practical cell in 1800, and it was Volta's cell that led to the work of Davy and Faraday.
Consider the electrochemical cell shown in Figure 1-1. Pieces of zinc and copper are immersed, respectively, in solutions of ZnSO4 and CuSO4. The solutions are in contact through a glass frit which is sufficiently porous to allow ions to pass through, but which will prevent mixing of the solutions. When a voltmeter is attached between the Zn and Cu electrodes, it is found that an electric potential has developed.
If the voltmeter is replaced by a resistor and an ammeter, it is found that an electric current flows. If, after current has flowed for some time, the electrodes are removed and weighed, it is found that the Zn electrode has lost mass and the Cu electrode has gained mass.
The electrode mass changes are as expected from Faraday's laws if the processes at the Zn and Cu electrodes are the half-reactions:

The overall cell reaction, is a spontaneous process, ΔG° = -212.8 kJ, and it is this spontaneous process which leads to the electric potential.
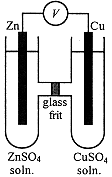
Figure 1-1. A galvanic cell.
An electrochemical cell generates a potential difference E. (The symbol E, commonly used in electrochemistry, refers to electromotive force, an archaic term for potential difference.) The electrical work done when n moles of electrons is passed by the cell can be found using Eq. (1-1), w = -nFE. It can be shown that the electrical work done by an electrochemical cell, at constant temperature and pressure, is equal to the change in Gibbs free energy of the cell components,
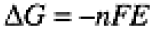 (1.1)
(1.1)
If the cell reaction is spontaneous, ΔG < 0 and E > 0, hence the negative sign in Eq. (1-1). Combining Eqs. (1-1) and (1-2), we have the Nernst equation, which relates E to the reaction quotient Q:
 (1.2)
(1.2)
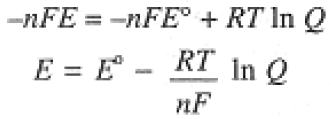 (1.3)
(1.3)
where E° = -DG°/nF is the standard potential of the cell, i.e., the potential of the cell when all species are in standard states. We sometimes use the Nernst equation with T = 298 K and the natural logarithm converted to base 10:
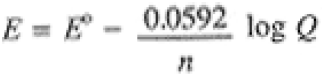 (1.4)
(1.4)
Example 1
Compute the potential of the cell shown in Figure 1-1 when T = 298 K, [Cu2+] = 0.010 M, [Zn2+] = 0.0050 M. The standard potential is computed from ΔG°,
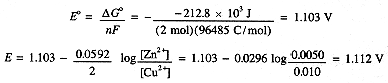
An electrochemical cell reaction, like any oxidation-reduction reaction, can be written as the sum of an oxidation half-reaction and a reduction half-reaction. In the case of a cell, these half-reactions correspond to the reactions at the two electrodes. Since the cell reaction is the sum of the half-cell reactions, it is convenient to think of dividing the cell potential into half-cell potentials. Unfortunately, there is no way of measuring a half-cell potential—we always nee two half-cells to make a cell, the potential of which is measurable. By convention, the half-cell reaction,
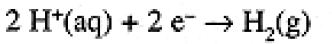 (1.5)
(1.5)
has been chosen as the primary reference half-cell with standard potential taken to be E° = 0.000 V. Standard half-cell potentials then are determined by cells that include Eq. (1-5) as one of the half-cell reactions. Thus the standard potential of the cell with cell reaction
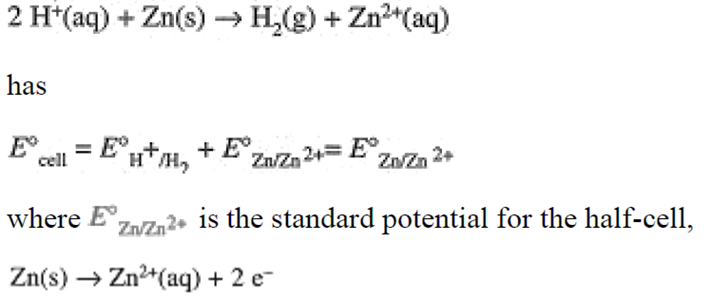
Standard half-cell potentials are usually tabulated as reduction potentials, e.g.,

The corresponding oxidation half-cell potential is the negative of this value, i.e., 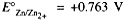 . Standard half-cell potentials can be used to compute standard cell potentials, standard Gibbs free energy changes, and equilibrium constants for oxidation reduction reactions.
. Standard half-cell potentials can be used to compute standard cell potentials, standard Gibbs free energy changes, and equilibrium constants for oxidation reduction reactions.
 الاكثر قراءة في الكيمياء الكهربائية
الاكثر قراءة في الكيمياء الكهربائية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















