
Faraday Laws of Electrolysis
 المؤلف:
Jerome L. Rosenberg and Lawrence M. Epstein
المؤلف:
Jerome L. Rosenberg and Lawrence M. Epstein
 المصدر:
College Chemistry
المصدر:
College Chemistry
 الجزء والصفحة:
p 134
الجزء والصفحة:
p 134
 18-7-2017
18-7-2017
 1154
1154
Faraday's Laws of Electrolysis
Chemists began passing electric current through solutions in the early 19th century; early results included the isolation of most of the alkali and alkaline earth metals by Humphry Davy. Davy's assistant, Michael Faraday, went on to study the quantitative aspects of electrolysis. By the 1830s,
Faraday had accumulated enough data to summarize his findings in two laws: (1) The mass of a substance liberated at or deposited on an electrode is proportional to the electric charge passed through the electrolyte. (2) The mass of such a substance is proportional to the molar mass of the substance, adjusted for the number of electrons required for the oxidation or reduction process. (Faraday's work was 40 years before the discovery of the electron so he worded the second law somewhat differently.)
The charge on 1 mol of electrons is the Faraday constant, 1F = 96485 C/mol. When current is passed through a solution of CuSO4, Cu2+ is reduced at the cathode and water is oxidized at the anode:
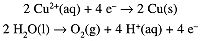
If 1.000F of charge is passed through the solution, 29.5g (0.500 mol) of metallic copper is deposited on the cathode and 8.00 g (0.250 mol) of O2(g) is liberated at the anode. In general, reduction occurs at the cathode and oxidation at the anode.
Faraday's laws can be summarized by:
• 1 faraday (F) = 96, 485 C/mol electrons;
• Mole relationships apply to electrons in
balanced oxidation-reduction half-reactions.
 الاكثر قراءة في الكيمياء الكهربائية
الاكثر قراءة في الكيمياء الكهربائية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


