

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Faraday Laws of Electrolysis
المؤلف:
Jerome L. Rosenberg and Lawrence M. Epstein
المصدر:
College Chemistry
الجزء والصفحة:
p 134
18-7-2017
1187
Faraday's Laws of Electrolysis
Chemists began passing electric current through solutions in the early 19th century; early results included the isolation of most of the alkali and alkaline earth metals by Humphry Davy. Davy's assistant, Michael Faraday, went on to study the quantitative aspects of electrolysis. By the 1830s,
Faraday had accumulated enough data to summarize his findings in two laws: (1) The mass of a substance liberated at or deposited on an electrode is proportional to the electric charge passed through the electrolyte. (2) The mass of such a substance is proportional to the molar mass of the substance, adjusted for the number of electrons required for the oxidation or reduction process. (Faraday's work was 40 years before the discovery of the electron so he worded the second law somewhat differently.)
The charge on 1 mol of electrons is the Faraday constant, 1F = 96485 C/mol. When current is passed through a solution of CuSO4, Cu2+ is reduced at the cathode and water is oxidized at the anode:
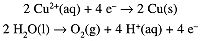
If 1.000F of charge is passed through the solution, 29.5g (0.500 mol) of metallic copper is deposited on the cathode and 8.00 g (0.250 mol) of O2(g) is liberated at the anode. In general, reduction occurs at the cathode and oxidation at the anode.
Faraday's laws can be summarized by:
• 1 faraday (F) = 96, 485 C/mol electrons;
• Mole relationships apply to electrons in
balanced oxidation-reduction half-reactions.
 الاكثر قراءة في الكيمياء الكهربائية
الاكثر قراءة في الكيمياء الكهربائية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















