
Calculating weight, particles, and moles
 المؤلف:
John T. Moore, EdD
المؤلف:
John T. Moore, EdD
 المصدر:
Chemistry Essentials For Dummies
المصدر:
Chemistry Essentials For Dummies
 الجزء والصفحة:
p 128
الجزء والصفحة:
p 128
 18-1-2017
18-1-2017
 1105
1105
Calculating weight, particles, and moles
The mole is the bridge between the microscopic and macroscopic world: 6.022 × 1023 particles ↔ 1 mole ↔ atomic/formula weight in grams
If you have any one of the three things — particles, moles, or grams — then you can calculate the other two. For example, the weight of a water molecule is 18.015 amu.
Because a mole is the formula (or molecular) weight in grams of a compound, you can now say that the weight of a mole of water is 18.015 grams. You can also say that 18.015 grams of water contains 6.022 × 1023 H2O molecules, or a mole of water.
And the mole of water is composed of two moles of hydrogen and one mole of oxygen.
Suppose you want to know how many water molecules are in 5.50 moles of water. You can set up a problem like this:
5.50 mol × 6.022 × 1023 molecules/mol = 3.31 × 1024 molecules. Or suppose that you want to know how many moles are in 25.0 grams of water. You can set up the problem like this:
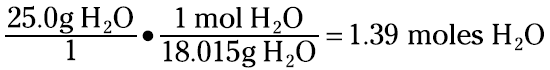
You can even go from grams to particles by going through the mole. For example, how many molecules are in 100.0 grams of carbon dioxide? The first thing you have to do is determine the molecular weight of CO2. Look at the periodic table to find that one carbon atom equals 12.011 amu and one oxygen atom weighs 15.000 amu. Now figure the molecular weight, like this:
[(1 × 12.011 g/mol) + (2 × 15.999 g/mol)] = 44.01 g/mol for CO2
Now you can work the problem: And going from particles to moles to grams is just as easy.
 الاكثر قراءة في الكيمياء الكهربائية
الاكثر قراءة في الكيمياء الكهربائية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


