

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Resonance Electronic Effects on Acid/Base Strength
المؤلف:
ADAM RENSLO
المصدر:
the organic chemistry of medicinal agent
الجزء والصفحة:
p260
12-7-2016
2526
Resonance Electronic Effects on Acid/Base Strength
In another essay we saw how resonance delocalization imparts partial double-bond character to amide bonds and affords special stabilization in aromatic ring systems like benzene. Not surprisingly, resonance delocalization of electrons plays a very important role in modulating the strengths of acids and bases as well. There are two major ways this can occur. The first is when lone pair electrons belonging to the basic X atom are delocalized and thus withdrawn into a larger π system (tending to make the X–H bond more acidic since :X− is stabilized). The second case is when another atom donates electrons into a π system that involves the basic X atom (tending to make the X–H bond less acidic since :X− is destabilized). We will see examples of both cases below, although the former scenario of electron withdrawal is more common than the latter.
As a first example of the effects of electron withdrawal through resonance delocalization, consider the trend in pKa values for several O–H containing functional groups (Figure 1.1). Each of the O–H bonds is potentially acidic, but the O atoms are attached in different molecular contexts allowing variable amounts of resonance electron delocalization and large differences in the relative acidities of the O–H bonds.
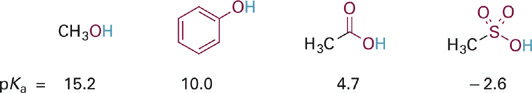
Figure 1.1 Example of effect of increasing resonance delocalization in functional groups with the same type of acidic X–H bond. In this figure and those that follow, atoms directly involved in resonance delocalization are shown in red.
Moving from left to right in this series the acids become stronger and the conjugate bases become weaker (less basic) as the negative charge becomes more spread out. In the conjugate base of methanol (i.e., the methoxide anion, CH3O−), the negative charge remains localized on the O atom and hence the basicity is similar to the basicity observed in HO− (pKa for H2O = 15.7).
Next in the series is phenol, the conjugate base of which is the phenoxide anion. The resonance hybrid structure of phenoxide (Figure 1.2) shows how the negative charge is distributed between the oxygen atom and the para- and ortho-carbon atoms by resonance delocalization. Since oxygen is more electronegative than carbon, the oxygen of phenoxide still carries the largest fraction of negative charge. However, the fractional charge on oxygen is much less in phenoxide as compared to methoxide and thus is less attractive to a proton (less basic). Comparing the pKa values (and recalling that each ΔpKa of 1 equals a 10-fold difference in relative basicity/acidity), the phenoxide anion is more than 100,000-fold less basic than the methoxide anion.
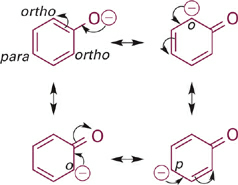
Figure 1.2 Resonance structures for phenoxide anion showing distribution of negative charge from O onto ortho- and para-carbon atoms of the phenyl ring.
Next in our series is acetic acid (pKa = 4.7), the conjugate base of which is the acetate anion (Figure 1.3). In acetate, the negative charge is delocalized onto two equally electronegative oxygen atoms, since the two resonance structures are identical. Each oxygen atom carries only 50% of the negative charge, which further stabilizes the anion and weakens the attraction to a proton. Comparing pKa values, this equal distribution of negative charge on two identical electronegative atoms makes the acetate anion more than 10 billion-fold less basic than methoxide anion and more than 100,000-fold less basic than phenoxide anion. This concept is extended further in the sulfonate anion (Figure 1.4) in which the negative charge is distributed equally among three oxygen atoms. Partial charge on each oxygen atom is reduced to just ~33%, affording the least “proton-seeking” conjugate base in the series.
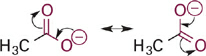
Figure 1.3 Resonance structures of acetate anion showing equal distribution of negative charge on the two oxygen atoms.
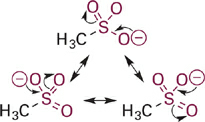
Figure 1.4 Resonance structures for methylsulfonate anion with the negative charge distributed equally among three oxygen atoms.
To summarize, delocalization of electrons such that the negative charge is spread out among many atoms lowers the fractional charge on the X atom, which lowers its basicity and increases the acidity of its conjugate acid (X–H). Likewise, delocalizing electrons onto more electronegative atoms like oxygen is more effective at lowering basicity than delocalization onto less electronegative atoms like carbon.
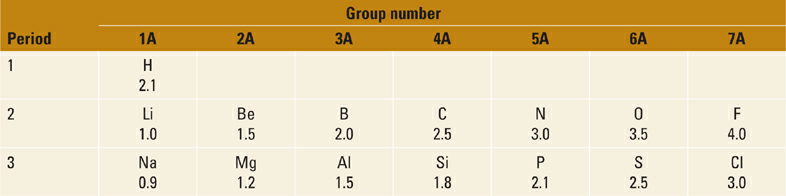
Consider now some similar trends in acids based on nitrogen, that is, N–H acids (Figure 1.5). Consistent with the difference in electronegativity between N and O, each of the N–H acids in Figure 1.5 is substantially weaker than the corresponding O–H acids in Figure 1.1. As with the oxygen acids, we see the same trend of increasing acidity of the N–H bond (and decreasing basicity of :N−) with increased resonance delocalization. Note, however, that the magnitude of change in acidity (and basicity) is significantly greater for the N–H acids. For example, we observe a much larger difference in acidity for aniline and methylamine (~13 pKa units, or 1013-fold) than we saw between phenol and methanol (5 pKa units, or 105-fold). This difference between N and O bases can be understood in terms of electronegativity. Since N and C are closer in electronegativity than O and C (Table 1.1), the ortho- and para-carbon atoms in the aniline anion (PhNH−) share a larger proportion of the negative charge with N than the analogous carbon atoms in phenoxide share with O.
Table 1.1 Electronegativities of Elements in the First Three Rows of the Periodic Table.
Similarly, resonance delocalization in the acetamide anion results in >50% of the negative charge residing on the more electronegative O atom. This effect is greater than in the analogous case of acetate (Figure 1.3), where the charge is equally shared between the two oxygen atoms. Similar analyses can be extended to the other N–H acids in Figure 1.5 and for C–H type acids as well.
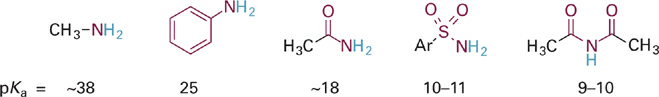
Figure 1.5 Comparison of acidities for neutral N–H acids shows same trend of increasing acidity with increasing electron delocalization, especially onto more electronegative atoms.
To summarize then, the stabilization of X− anions by resonance delocalization leads to a weaker base and to more acidic X–H bonds. Moreover, these effects are stronger in cases where the charge can be shared by atoms of greater electronegativity than the X atom.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















