

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Alkyl Groups
المؤلف:
John McMurry
المصدر:
Organic Chemistry
الجزء والصفحة:
9th - p70
25-3-2016
8827
Alkyl Groups
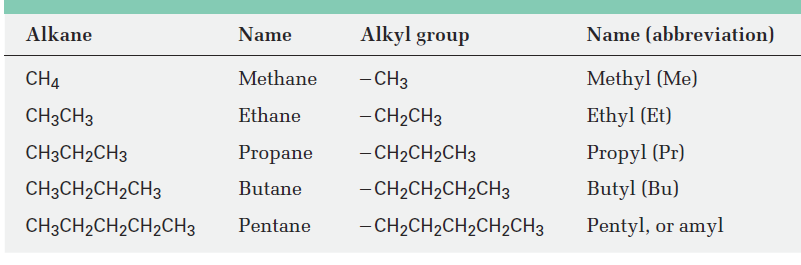
If you imagine removing a hydrogen atom from an alkane, the partial structure that remains is called an alkyl group. Alkyl groups are not stable compounds themselves, they are simply parts of larger compounds. Alkyl groups are named by replacing the -ane ending of the parent alkane with an -yl ending. For example, removal of a hydrogen from methane, CH4, generates a methyl group, -CH3, and removal of a hydrogen from ethane, CH3CH3, generates an ethyl group, -CH2CH3. Similarly, removal of a hydrogen atom from the end carbon of any straight-chain alkane gives the series of straight-chain alkyl groups shown in Table 1. Combining an alkyl group with any of the functional groups listed earlier makes it possible to generate and name many thousands of compounds. For example:
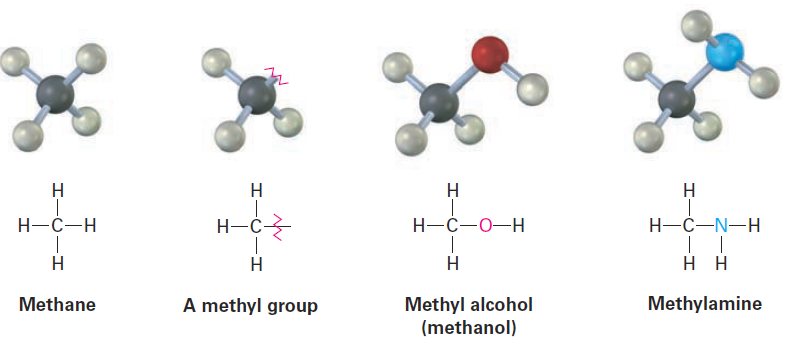
Table 1: Some Straight-Chain Alkyl Groups
Just as straight-chain alkyl groups are generated by removing a hydrogen from an end carbon, branched alkyl groups are generated by removing a hydrogen atom from an internal carbon. Two 3-carbon alkyl groups and four 4-carbon alkyl groups are possible.
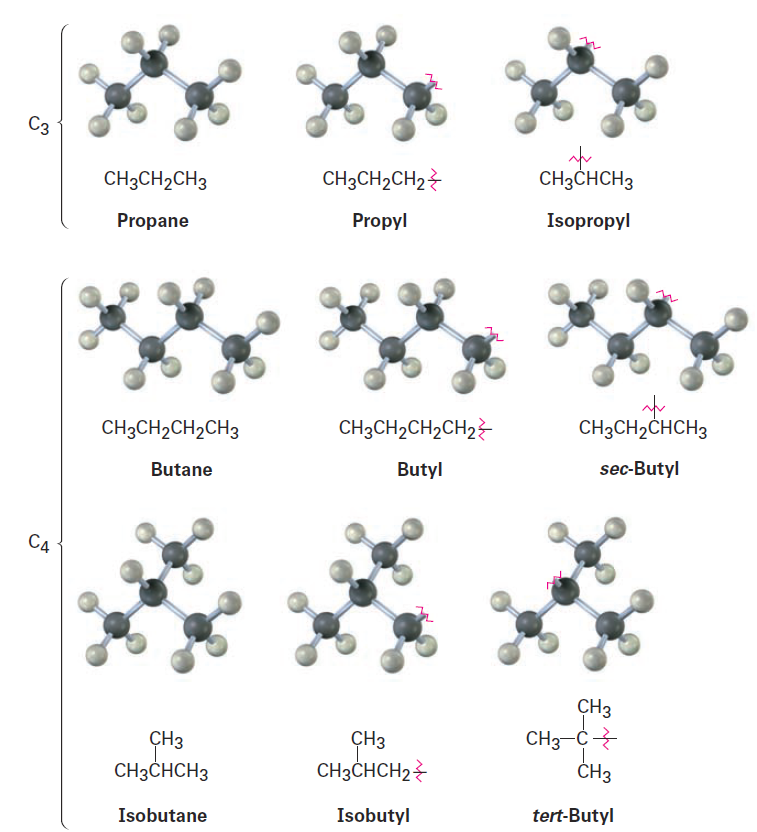
One further comment about naming alkyl groups: the prefixes sec- (for secondary) and tert- (for tertiary) used for the C4 alkyl groups in Figure above refer to the number of other carbon atoms attached to the branching carbon atom. There are four possibilities: primary (1°), secondary (2°), tertiary (3°), and quaternary (4°).
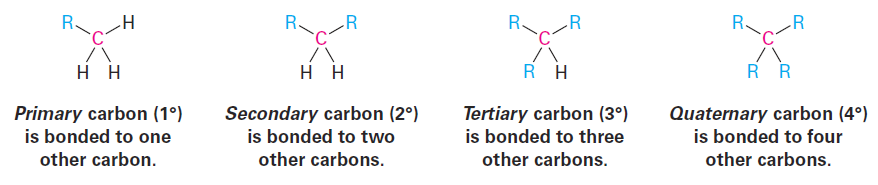
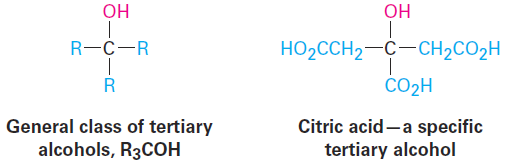
The symbol R is used here and throughout organic chemistry to represent a generalized organic group. The R group can be methyl, ethyl, propyl, or any of a multitude of others. You might think of R as representing the Rest of the molecule, which isn’t specified. The terms primary, secondary, tertiary, and quaternary are routinely used in organic chemistry, and their meanings need to become second nature. For example, if we were to say, “Citric acid is a tertiary alcohol,” we would mean that it has an alcohol functional group (- OH) bonded to a carbon atom that is itself bonded to three other carbons. (These other carbons may in turn connect to other functional groups.)
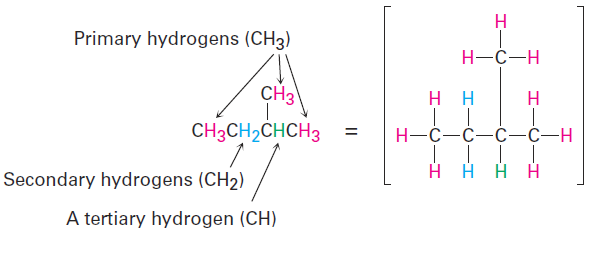
In addition, we also speak of hydrogen atoms as being primary, secondary, or tertiary. Primary hydrogen atoms are attached to primary carbons (RCH3), secondary hydrogens are attached to secondary carbons (R2CH2), and tertiary hydrogens are attached to tertiary carbons (R3CH). There is, of course, no such thing as a quaternary hydrogen. (Why not?)
 الاكثر قراءة في الهايدروكاربونات
الاكثر قراءة في الهايدروكاربونات
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















