
Electron configurations
 المؤلف:
John T. Moore, EdD
المؤلف:
John T. Moore, EdD
 المصدر:
Chemistry Essentials For Dummies
المصدر:
Chemistry Essentials For Dummies
 الجزء والصفحة:
p29
الجزء والصفحة:
p29
 15-3-2016
15-3-2016
 2419
2419
Electron configurations
Energy level diagrams are useful when you need to figure out chemical reactions and bonding, but they’re very bulky to work with. Wouldn’t it be nice if there were another representation that gives just about the same information but in a much more concise form? Well, there is. It’s called the electron configuration.
The electron configuration for oxygen is 1s22s22p4. Compare that notation with the energy level diagram for oxygen in Figure 1.. Doesn’t the electron configuration take up a lot less space? You can derive the electron configuration from the energy level diagram. The first two electrons in oxygen fill the 1s orbital, so you show it as 1s2 in the electron configuration.
The 1 is the energy level, the s represents the type of orbital, and the superscript 2 represents the number of electrons in that orbital. The next two electrons are in the 2s orbital, so you write 2s2. And finally, you show the four electrons in the 2p orbital as 2p4. Put it all together, and you get 1s22s22p4. The sum of the superscript numbers equals the atomic number, or the number of electrons in the atom.
Here are a couple of electron configurations you can use to check your conversions from energy level diagrams:
Chlorine (Cl): 1s22s22p63s23p5
Iron (Fe): 1s22s22p63s23p64s23d6
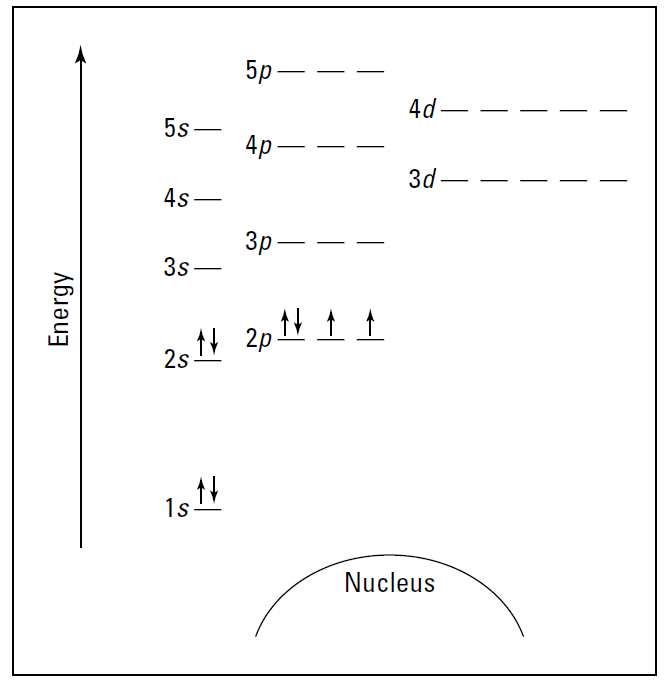
Figure 1: Energy level diagram for oxygen.
 الاكثر قراءة في مقالات متنوعة في علم الكيمياء
الاكثر قراءة في مقالات متنوعة في علم الكيمياء
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


