

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Acids and Bases: The Lewis Definition
المؤلف:
John McMurry
المصدر:
Organic Chemistry
الجزء والصفحة:
p50
10-3-2016
4846
Acids and Bases: The Lewis Definition
The Lewis definition of acids and bases is more encompassing than the Brønsted–Lowry definition because it’s not limited to substances that donate or accept just protons. A Lewis acid is a substance that accepts an electron pair, and a Lewis base is a substance that donates an electron pair. The donated electron pair is shared between the acid and the base in a covalent bond.
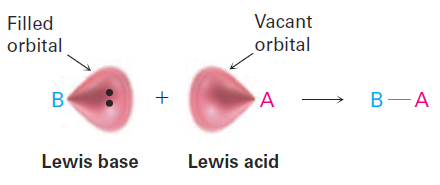
Lewis Acids and the Curved Arrow Formalism
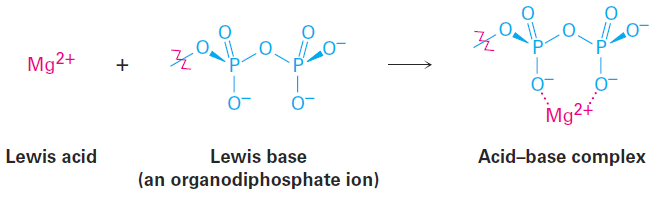
The fact that a Lewis acid is able to accept an electron pair means that it must have either a vacant, low-energy orbital or a polar bond to hydrogen so that it can donate H+ (which has an empty 1s orbital). Thus, the Lewis definition of acidity includes many species in addition to H+. For example, various metal cations, such as Mg2+, are Lewis acids because they accept a pair of electrons when they form a bond to a base. We’ll also see in later chapters that certain metabolic reactions begin with an acid–base reaction between Mg2+ as a Lewis acid and an organic diphosphate or triphosphate ion as the Lewis base.
In the same way, compounds of group 3A elements, such as BF3 and AlCl3, are Lewis acids because they have unfilled valence orbitals and can accept electron pairs from Lewis bases, as shown in Figure1. Similarly, many transitionmetal compounds, such as TiCl4, FeCl3, ZnCl2, and SnCl4, are Lewis acids.
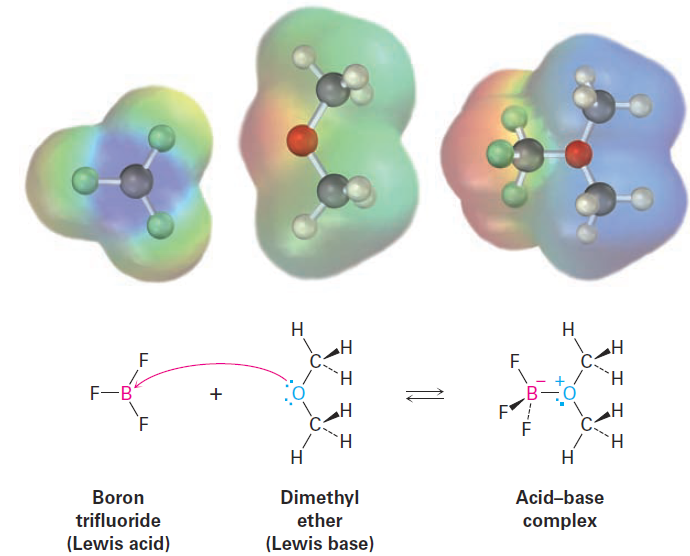
Figure 1. The reaction of boron trifluoride, a Lewis acid, with dimethyl ether, a Lewis base. The Lewis acid accepts a pair of electrons, and the Lewis base donates a pair of nonbonding electrons. Note how the movement of electrons from the Lewis base to the Lewis acid is indicated by a curved arrow. Note also how, in electrostatic potential maps, the boron becomes more negative (red) after reaction because it has gained electrons and the oxygen atom becomes more positive (blue) because it has donated electrons.
Look closely at the acid–base reaction in Figure 1, and note how it is shown. Dimethyl ether, the Lewis base, donates an electron pair to a vacant valence orbital of the boron atom in BF3, a Lewis acid.. A curved arrow always means that a pair of electrons moves from the atom at the tail of the arrow to the atom at the head of the arrow. We’ll use this curved-arrow notation throughout the remainder of this text to indicate electron flow during reactions. Some further examples of Lewis acids follow:
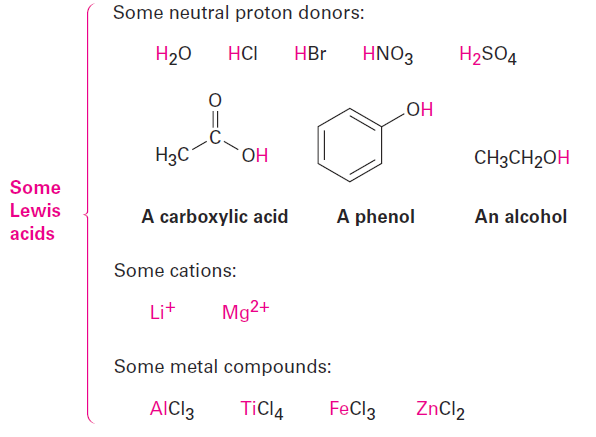
Lewis Bases
The Lewis definition of a base—a compound with a pair of nonbonding electrons that it can use to bond to a Lewis acid—is similar to the Brønsted–Lowry definition. Thus, H2O, with its two pairs of nonbonding electrons on oxygen, acts as a Lewis base by donating an electron pair to an H1 in forming the hydronium ion, H3O+.
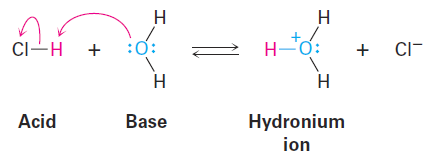
In a more general sense, most oxygen- and nitrogen-containing organic compounds can act as Lewis bases because they have lone pairs of electrons. A divalent oxygen compound has two lone pairs of electrons, and a trivalent nitrogen compound has one lone pair. Note in the following examples that some compounds can act as both acids and bases, just as water can. Alcohols and carboxylic acids, for instance, act as acids when they donate an H+ but as bases when their oxygen atom accepts an H+.
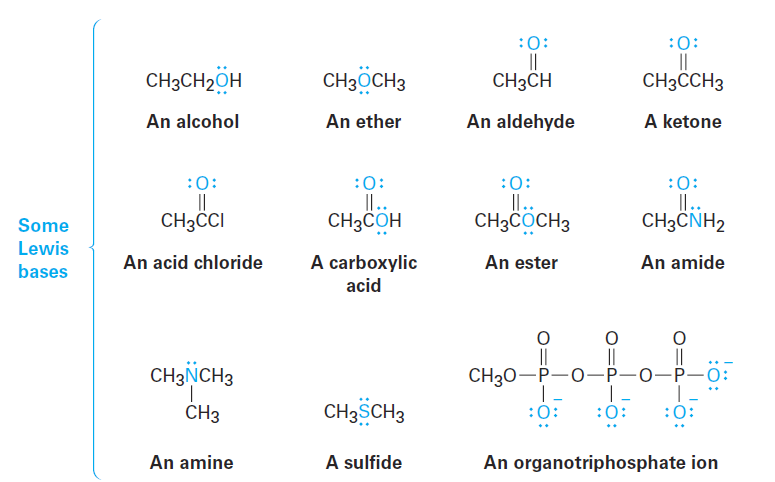
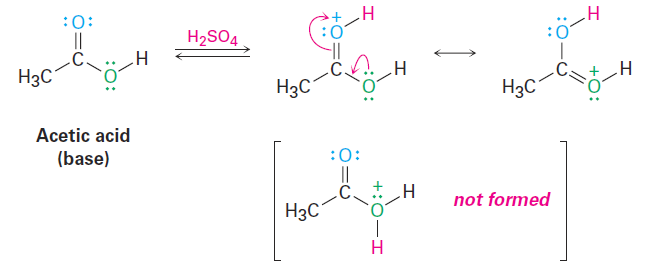
Notice in the list of Lewis bases just given that some compounds, such as carboxylic acids, esters, and amides, have more than one atom with a lone pair of electrons and can therefore react at more than one site. Acetic acid, for example, can be protonated either on the doubly bonded oxygen atom or on the singly bonded oxygen atom. Reaction normally occurs only once in such instances, and the more stable of the two possible protonation products is formed. For acetic acid, protonation by reaction with sulfuric acid occurs on the doubly bonded oxygen because that product is stabilized by two resonance forms.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















