
تاريخ الفيزياء

علماء الفيزياء


الفيزياء الكلاسيكية

الميكانيك

الديناميكا الحرارية


الكهربائية والمغناطيسية

الكهربائية

المغناطيسية

الكهرومغناطيسية


علم البصريات

تاريخ علم البصريات

الضوء

مواضيع عامة في علم البصريات

الصوت


الفيزياء الحديثة


النظرية النسبية

النظرية النسبية الخاصة

النظرية النسبية العامة

مواضيع عامة في النظرية النسبية

ميكانيكا الكم

الفيزياء الذرية

الفيزياء الجزيئية


الفيزياء النووية

مواضيع عامة في الفيزياء النووية

النشاط الاشعاعي


فيزياء الحالة الصلبة

الموصلات

أشباه الموصلات

العوازل

مواضيع عامة في الفيزياء الصلبة

فيزياء الجوامد


الليزر

أنواع الليزر

بعض تطبيقات الليزر

مواضيع عامة في الليزر


علم الفلك

تاريخ وعلماء علم الفلك

الثقوب السوداء


المجموعة الشمسية

الشمس

كوكب عطارد

كوكب الزهرة

كوكب الأرض

كوكب المريخ

كوكب المشتري

كوكب زحل

كوكب أورانوس

كوكب نبتون

كوكب بلوتو

القمر

كواكب ومواضيع اخرى

مواضيع عامة في علم الفلك

النجوم

البلازما

الألكترونيات

خواص المادة


الطاقة البديلة

الطاقة الشمسية

مواضيع عامة في الطاقة البديلة

المد والجزر

فيزياء الجسيمات


الفيزياء والعلوم الأخرى

الفيزياء الكيميائية

الفيزياء الرياضية

الفيزياء الحيوية

الفيزياء العامة


مواضيع عامة في الفيزياء

تجارب فيزيائية

مصطلحات وتعاريف فيزيائية

وحدات القياس الفيزيائية

طرائف الفيزياء

مواضيع اخرى
ELECTRON SPIN
المؤلف:
Mark Csele
المصدر:
FUNDAMENTALS OF LIGHT SOURCES AND LASERS
الجزء والصفحة:
p65
9-3-2016
2140
ELECTRON SPIN
Continuing with the Bohr analogy, if we consider the electron to be orbiting the nucleus like a miniature planet, that electron will also have a spin associated with it. In a classical view, a spinning charge produces a magnetic moment that interacts with other magnetic fields, but this model fails to account for the quantized nature of this spin (for which experimental evidence is provided in the form of the Stern Gerlach experiment), so it is a purely quantum concept, despite the fact that the name suggests a classical foundation.
Spin is not, unto itself, important (spectroscopically speaking). What affects energy levels is the way in which spin interacts with angular orbital momentum in what is called l-s coupling (where the s represents spin). The spin of an electron can assume two possible values, -½ and +½ . When spin is added and subtracted from orbital angular momentum (l), the effects of spin on energy levels can be seen. Consider Figure 1.1, which shows (in a Bohr model type of approach) an orbiting electron. Depending on the direction of the spin of the electron, the energy level of that electron will change. When the orientation of the spin momentum is in the same direction as the orbital angular momentum, the resulting energy level I slightly higher than when the orientation of the two momentums is in the opposite
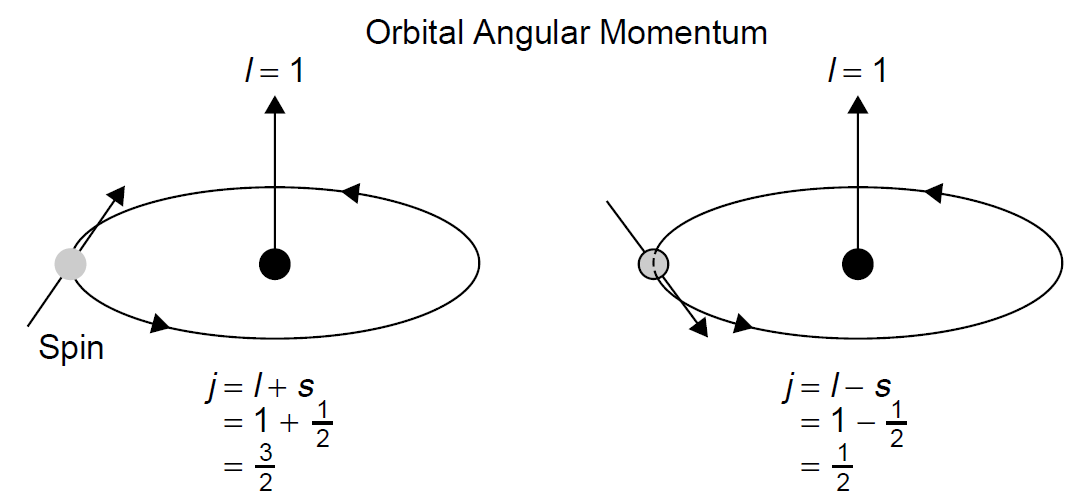
Figure 1.1. Electron spin and l–s coupling.
direction. This effect is designated by a subscript j, which is a combination of l and s. In this case, l = 1, so j can assume values of 1/2 or 3/2 , depending on the orientation of spin relative to orbital angular momentum.
The effect of spin on energy levels can be quite small but is evident in the hydrogen spectrum. When a hydrogen line such as the red line at 656.3 nm is examined using high resolution spectroscopy, the “single” line is actually found to be a doublet of two very closely spaced lines, separated by only about 0.02 nm (most spectrographs lack enough resolution to discern the individual lines). The transition is commonly referred to as the n = 3 to n = 2 transition in hydrogen. The actual lower state for the transition (n =2) is found to be two energy states very close together, with electron spins in opposite directions. The slightly higher energy state results from the electron spinning in the same direction as the orbital angular momentum, the lower state where spin is opposite, as shown in Figure 1.2.
The designation of the 2P levels is determined by adding l+ s and l - s. The subscripts are determined in the same manner as in Figure 1.1. The resulting levels are then designated as 2P3/2 and 2P1/2. Called a doublet, this results in the production of two closely spaced spectral lines (doublets occur in P orbitals in an atom where there is a single valence electron, such as hydrogen or sodium). Note that j is an absolute value, and should a negative number result, the negative sign is simply ignored.
The effect of the spin of electrons on the energy levels is most dramatic in a multi electron atom such as helium, which has two electrons in the outer shell. If the spins of each electron are parallel, the resulting energy levels will be lower than if the spins are opposing. The reason for the behavior is that electrons with parallel spins have a greater chance of being closer together than if their spins are opposite. The closer together the electrons are, the higher the resulting energies. (When two negative charges oppose each other, a higher energy will result.) Transitions between otherwise identical atoms would show higher energies in an atom having electrons with parallel spins than in an atom with opposing spins. Called the spin-spin effect, this serves to split energy levels. It is responsible for splitting of the yellow sodium D lines, which unlike the hydrogen lines, which are 0.02 nm apart, are 0.6 nm apart and easily discerned with an inexpensive diffraction grating.
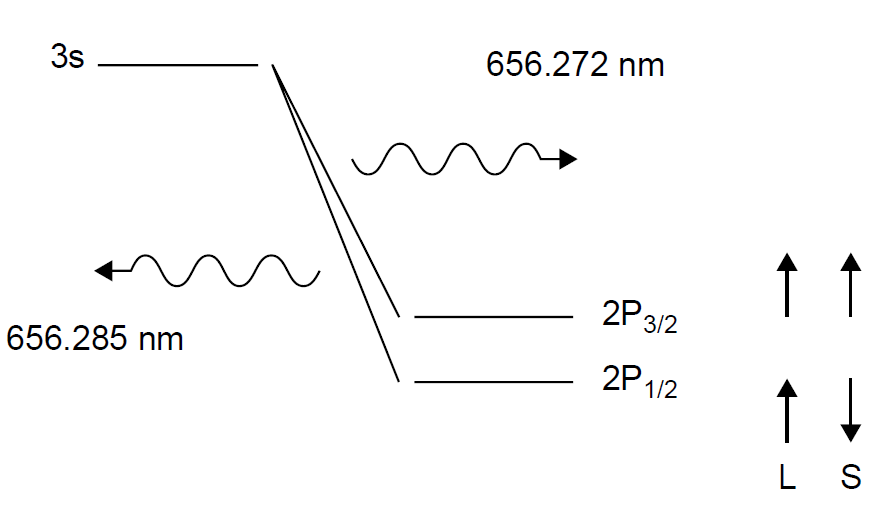
Figure 1.2. Hydrogen fine structure.
 الاكثر قراءة في ميكانيكا الكم
الاكثر قراءة في ميكانيكا الكم
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















