


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 2025-03-17
Date: 7-3-2021
Date: 3-12-2015
|
Caged ATP
The synthesis of caged ATP (caged ADP and caged phosphate) and many of the properties that make these reagents useful in a variety of biological applications were first published in 1978 (1). The driving force for the development of such a reagent was the desire to be able to introduce ATP rapidly (and synchronously) at sites of biological interest at a desired time. A pulse of ATP would be released by light activation and would then initiate the processes being studied (Fig. 1). In contrast to affinity labeling, here the ATP analogue should not bind to its site prior to activation and ATP is released freely in solution. The strategy employed was an approach that had previously been used in synthetic organic chemistry (ie, photodeprotection). In the chemical applications, a desired functionality in a molecule was modified and protected from a variety of reagents and conditions during a multistep synthesis, to be deprotected at a later step by a light-activated process. In biological applications, the caged substrates are “protected” from their receptor, enzyme, or binding site by the chromophoric moiety, and following the pulse of light they are released to their biological target.
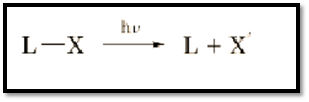
Figure 1. Basis of photorelease of a caged substrate. The biological ligand (L) or substrate is rendered inactive by the attachment of a photocleavable chromophore (x). Following photoactivation the ligand is released accompanied by the modified chromophore, or photofragment.
The properties of molecules appropriate for the study of biological systems are subject to more constraints than those used in synthetic organic chemistry. Much of the subsequent work on various caged ATP molecules and other caged compounds has been aimed at refining or improving their basic properties to expand the areas of application or overcome a particular limitation. The design requirements for a caged ATP are illustrative of the needs of any caged biological ligand or substrate (2) .
The basic requirements for a biologically useful caged ATP (and for most other caged compounds( are as follows: (i) The activating light must be at wavelengths long enough to avoid damage to biological material (usually greater than 300 nm). (ii) The photorelease process must be as efficient as possible; that is, the quantum yield (ratio of molecules of product obtained to molecules of caged compound excited) must be as high as possible. (iii) The photorelease process should be as rapid as possible, relative to the rate of the process of interest; this has been achieved in the tens of microseconds to millisecond range, at normal temperatures and pH values. (iv) The photoreleased fragment, which usually is the modified chromophore, should not be harmful to biological materials ) see text below). (v) The absorbance of the caged compound should be reasonably high at the exciting wavelength or wavelength range. Initially it was thought that too high an absorbance might lead to nonuniform release of substrate across the depth of an illuminated sample. Interestingly, for the recent application of multiphoton excitation, very high absorbances are an advantage. (vi) Prior to photolysis, the caged substrate should neither bind to nor interact with the biological material of interest. In many respects, the first caged ATP reported and subsequently used (but the second synthesized) fulfilled many of these requirements. In a variety of systems, one or other of these properties has been less than ideal, and this has led to various variations on the original structure in attempts to provide an improved caged ATP.
1. Synthesis and Photochemical Properties of Caged ATP
The initial synthesis of caged ATP was based on a strategy of first preparing caged phosphate and then coupling this to ADP. This had the advantage that the photochemical lability, quantum yield, and so on, could be first characterized, using caged phosphate and the readily assayed free substrate, inorganic phosphate (1). This route suffered from the following disadvantages: (i) It was less direct than was desirable to achieve a gamma 32P-labeled caged ATP for a variety of phosphorylation studies, and (ii) it was not generally applicable to a wide range of phosphate-bearing biological ligands that might be desirable to cage. It would be better to have a more universal caging moiety that could be readily attached to the nucleotide or other organophosphates. These disadvantages were overcome by the development of a synthesis by Trentham and co-workers, who were able to attach the protecting 2-nitrobenzyl-based chromophore directly to the terminal phosphate of ATP, using a diazoprecursor (3). This was based on an approach that had been used by others to prepare a photosensitive cyclic AMP phosphotriester analogue (4). This method was used to prepare a wide array of caged phosphorylated biological ligands, including GTP, ADP, inositol trisphosphate, and so on (3).
The quantum yield for the first successful caged ATP was 0.54 and has not been bettered by subsequent analogues. The first caged ATP that was synthesized, the primary 2-nitrobenzyl analogue, yielded on photolysis some ATP, but only very low levels, even though photolysis was complete. It was hypothesized that this was due to a reaction between ATP and the released photofragment (2-nitrosobenzaldehyde), so that a chemically modified ATP resulted (1). The 2-nitrophenylethyl analogue (from the secondary benzyl alcohol) then became the molecule of choice, because photolysis yielded the less reactive 2-nitrosoacetophenone and free ATP in high yield (Fig. 2). This is the caged ATP that has been used most frequently in biological studies. The absorbance properties of caged ATP are simply the sum of the spectra of the 2-nitrobenzyl moiety and of ATP. This results in a long tail of absorbance that extends to above 350 nm. This has enabled illumination above 300 nm to be employed (using lasers or flash-lamps) so that photodamage due to absorption by most biological samples is avoided.
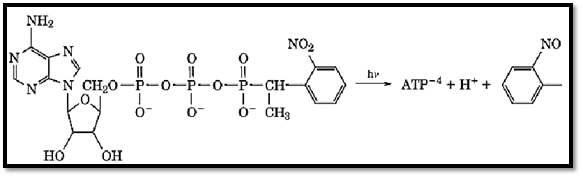
Figure 2. Photolysis of caged ATP. Photolysis of caged ATP produces a proton, free ATP, and the photofragment, 2-nitrosoacetophenone.
2. Mechanism and Kinetics of Photorelease
Although the earliest studies showed that ATP release could be achieved in a subsecond time frame, ideas about the kinetics of the release process and the likely mechanism were initiated by the report of McCray et al. in 1980 (5). From this work it was apparent that the rate of release of ATP from caged ATP was pH-dependent and in the millisecond time range.
Importantly, it was also pointed out in this work that there was a chromophoric intermediate on the reaction pathway, identified tentatively as an aci-nitro intermediate. The intermediacy of this type of chromophore has proven useful in later mechanistic studies of the photorelease of caged substrates, because it is central to almost all photorelease processes in which the protecting moiety is a 2-nitrobenzyl residue. Another tool that has been useful in mechanistic studies, and profitably used by Trentham and colleagues, is the reaction of the photoreleased nitrosoketone with thiol groups. This reaction had been initially employed by Kaplan et al. (1) to protect biological samples against any damaging effects of the photofragment, because it was known that nitrosoketones react readily with thiols. It was shown that the inhibition of the Na,K-ATPase enzyme due to the actions of the photofragment could be prevented by the simultaneous presence of dithiothreitol (DTT) or similar reagents. Furthermore, the protecting thiol had to be present in amounts at least stoichiometric with the released photofragment. In many subsequent applications, it has become routine to include reduced glutathione or DTT in the reaction media to mop up the released photofragment. Trentham and colleagues (6) used the rapid rate of this reaction to examine the release rates of the photofragment. This proved valuable because with caged ATP, as with many photoreleased biological substrates, it is often difficult to identify a biological process that is sufficiently fast to be used unambiguously as a bioassay to determine the photorelease rate of substrate. It should be emphasized, however, that for all new caged substrates it is essential to measure the release rate of the photoproduct of interest and not merely the rate of decay of an intermediate on the release pathway. There have been several reports of biphasic decay of putative aci-nitro intermediates when their breakdown was followed using transient ultraviolet spectroscopy (7, 8).
The most recent estimates (obtained from time-resolved infrared spectroscopy) of the formation of free ATP following the photolysis of caged ATP are about 220 s–1 at pH 7 and 22°C, a rate that is the same as the aci-nitro anion intermediate decay rate (9). An outline of the steps involved in the photolysis pathway are shown in Figure 3.
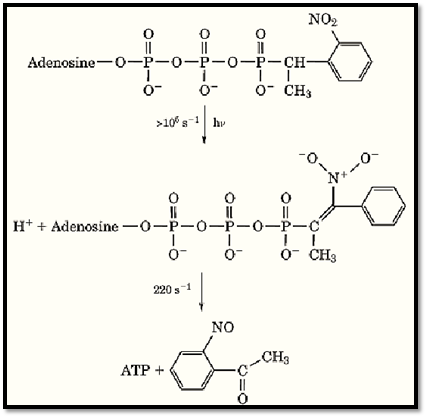
Figure 3. Scheme of breakdown of caged-ATP following excitation. The release rate of ATP is controlled by dark reactions that follow photoexcitation and relaxation to ground states. A detailed study of the products and intermediates has been made using time-resolved infrared spectroscopy and isotopomers of caged ATP (6).
3. Other Caged ATP Structures
The most familiar caged ATP is P3-(1-(2-nitrophenyl)ethyl) adenosine 5′-triphosphate. Variations on the basic theme have been made in the hope of increasing the long wavelength absorbance of the molecule or of speeding the rate of release. Unfortunately, no molecule yet reported has achieved this aim while simultaneously maintaining the relatively high quantum yield. Most attempts have involved variations in the chromophore, while maintaining the same nitrobenzyl photochemical cleavage mechanism (10). Recently potentially useful alternative photochemistries have begun to be exploited, but as yet no generally useful molecule has emerged (11-13).
4. Biological Applications of Caged ATP
The two areas where caged ATP has been used most extensively are in studying the mechanism of active transport by ion pumps and in the mechanism of muscle contraction and its regulation. In single turnover studies of the Na,K-ATPase (see text below), Forbush showed that caged ATP did in fact bind with low affinity to the enzyme prior to photolysis (14). This leads to extra complications in kinetic modeling of events following photolysis. Subsequently, similar effects have been noted in muscle fibers (15). Such observations suggest that a caged ATP with a bulkier chromophore might reduce the pre-photolysis binding.
5. Studies on Ion Pumps
The ion pumps are P-type ATPases that couple the transport of ions against their electrochemical potential gradient to the hydrolysis of ATP. This family of membrane proteins includes the Na pump or Na,K-ATPase, the Ca pump [from intracellular membranes (SERCA) or plasma membranes ) PMCA)], proton pumps from a variety of organisms such as yeast, bacteria, and so on, and a range of heavy metal pumps that transport Cu, Cd, Ni, and so on, across cellular membranes throughout the animal and plant kingdom (16). The studies on ion pumps that have used caged ATP illustrate well several of the different advantages of this experimental strategy. These proteins carry out a series of biochemical transformations that are thought to accompany the transport of cations across the membrane. These transformations arise from the intermediate involvement of a phosphoenzyme, which is formed by transferring the terminal phosphate of ATP to the protein at an early step in the cycle, and from the protein to water in a later step. These kinase and phosphatase activities are linked to ion binding, translocation, and ion release steps (17). In order to probe the cation activation of some of these processes, it was necessary in the case of the Na pump to be able to initiate the pump cycle in a sealed system. This was required because the activating and inhibitory cations have very asymmetric effects, depending on whether they act at the extracellular or intracellular surface.
The strategy employed was to trap caged ATP within resealed human erythrocyte ghosts. The extracellular medium could then be altered at will, and the Na pump process could be initiated by illumination of the ghost suspension and release of intracellular ATP from caged ATP. This enabled characterization of the side effects of activating and inhibiting cations on the transphosphorylation reactions under genuine initial rate conditions (18, 19). The essential properties of the photorelease strategy here were the stability of caged ATP during experimental procedures, until ultraviolet irradiation, when ATP could be released in good yield synchronously inside the cells in suspension. The possibility of releasing ATP from caged ATP in a rapid synchronous fashion within an ordered structure was also exploited in structural studies of the Ca pump in oriented multilayers where diffraction before and after the release of ATP from caged ATP showed movements of the protein mass relative to the membrane structure during the reaction cycle (20). Such studies would not have been possible without the photorelease approach.
Ion pumps transfer charge during their reaction cycle across cell membranes; and electrophysiological measurements of such movements, along with their analysis, have been a central area of study. In recent years, Bamberg and co-workers (21) and Apell and co-workers (22) have initiated the use of caged ATP in a novel experimental system to analyze the mechanistic consequences of these phenomena in a variety of ion pumps. These workers have used black lipid membranes and have either attached or fused to them biomembrane fragments or vesicles containing ion pumps. Following the rapid, synchronous release of ATP from caged ATP in the medium bathing the membranes, the current-carrying and charge translocating steps can be analyzed with a conventional electrophysiological measuring system (23, 24). Such studies have probed the basis of the electrogenic nature of the Na pump and of the electrically neutral basis of the gastric proton pump, for example (25). Recent studies have dissected out the electrical properties of the bacterial active K transporter, the Kdp-ATPase of Escherichia coli (26). 5.1. Regulation and Control of Muscle Contraction An adequate understanding of the mechanism of muscle contraction can only be achieved from experiments in a fairly intact and complex tissue. This is because the structure of the muscle fiber and its organization is an inherent feature of its function. The sliding filament model for the molecular basis of muscle contraction is the prevailing paradigm in this field, and its central feature is this relationship (27, 28). Thus it is necessary to be able to perform high-resolution kinetic studies in an ordered array of macromolecules. Recent efforts have been aimed at understanding the precise steps in the ATP hydrolysis cycle carried out by myosin that lead to the generation of force during contraction. Caged ATP has found application in detailed studies on the kinetics of ATP hydrolysis, structural changes in the fiber organization associated with contraction and relaxation (at the macroscopic and molecular levels), spectroscopic measurements on muscle fibers, and the process of relaxation associated with Ca pumping by the sarcoplasmic reticulum. Studies in all these areas have received considerable impetus by the introduction of the photorelease strategies. It has been shown that it is possible to photorelease ATP (or other substrates) in chemically skinned muscle fibers in the millisecond time range and to cause the synchronized initiation of biochemical processes that produce contraction (or relaxation) in the bundle of fibers (29, 30). Prior to this technology, such experiments were limited by the diffusional delays that were inherent in mechanically adding substrate and allowing it to diffuse into the fiber to its site of action. Initial studies that employed this approach to analyze the kinetics of the mechanical processes have now been greatly extended; by using covalently attached chromophoric reporters or paramagnetic reporters, the kinetics of conformational changes in the myosin protein can be monitored (31, 32). As well as such structural studies, it has also been possible to carry out time-resolved diffraction studies of fibers in a synchnotron beam prior to and following the release of caged ATP within the fiber (33, 34). These applications use the highly ordered skeletal muscle system to understand the basis of muscle contraction and relaxation. In smooth muscle, the contractile system has evolved to a highly complex level of cellular regulation, and many signal transducing systems and effectors play a role in regulating smooth muscle activity (35). In this area, the photorelease of caged ATP (and a variety of second messengers) continues to be a highly fruitful strategy. Recent studies by the Somlyos and their group provide a wide range of applications of this technology to the smooth muscle system (36,
37 ).
5.2. Other Systems
Since 1980 to the present time (early 1999) there have been more than 200 articles published using caged ATP, and many more using related substrates. These have extended from difference Fourier structural determinations of proteins with or without bound substrates (38, 39), to the cellular effects of occupancy of receptors (40). Structural biology of macromolecules, especially proteins, continues to provide enormous insights into molecular biological processes, and it is in this area that several interesting applications have great potential. The possibility of obtaining not merely a static picture of a protein at atomic resolution, but also one that contains dynamic information, is enticing. The accessibility of high-intensity radiation sources and a revival of interest in the Laue Diffraction method have made fast X-ray crystallography studies an attainable goal. The idea that such measurements could be made with protein crystals before and after laser-induced release of caged substrates within a crystal has received recent attention; and although many of the technical difficulties (uniformity of release, fate of photofragment, local heating effects) have not been overcome, this approach offers great promise for the future (41, 42). The number of physiological systems that have begun to be probed using caged ATP continues to increase; recent additions to this group include L-type Ca channel modulation (43), single kinesin molecules in optical traps (44), ATP-sensitive K channels in cholinergic interneurons (45), sea-urchin sperm flagella motility (46), and limulus photoreceptors (47).
6. Summary
The introduction of the photorelease strategy that followed the description of caged ATP has now been extended to a wide array of biological substrates, including nucleoside phosphates, cyclic AMP, cyclic GMP, protons, Ca2+, Mg2+, peptides, inositol phosphates, sugars, amino acids, neurotransmitters, toxins, and so on. In almost all cases, the photochemical process has the same basis as the original caged ATP strategy; along with a wide array of biological substrates, the approach can now be employed to probe systems that range in complexity from single protein crystals to brain slices.
References
1. J. H. Kaplan, B. Forbush III, and J. F. Hoffman (1978) Biochemistry 17, 1929–1935.
2. G. P. Hess (1999) (this encyclopedia).
3. J. W. Walker, G. P. Reid, J. A. McGray, and D. R. Trentham (1988) J. Am. Chem. Soc. 110, 7170-7177.
4. J. Engels and E.-J. Schlaeger (1977) J. Med. Chem. 20, 907–911.
5. J. A. McCray, L. Herbette, T. Kihura, and D. R. Trentham (1980) Proc. Natl. Acad. Sci. USA77 , 7237-7241.
6. A. Barth et al. (1997) J. Am. Chem. Soc. 119, 4149–4159.
7. J. E. T. Corrie (1993) J. Chem. Soc., Perkins Trans 1993, 2161–2166.
8. G. C. R. Ellis-Davies, J. H. Kaplan, and R. J. Barsotti (1996) Biophys. J. 70, 1006–1016.
9. A. Barth et al. (1995) J. Am. Chem. Soc. 117, 10311–10316.
10. J. F. Wootton and D. R. Trentham (1989) NATO ASI Series C 272, 277–296.
11. J. E. Baldwin et al. (1990) Tetrahedron 46, 6879–6884.
12. R. S. Givens et al. (1993) J. Am. Chem. Soc. 115, 6001–6012.
13. J. E. T. Corrie and D. R. Trentham (1992) J. Chem. Soc., Perkins Trans I, 2409–2417.
14. B. Forbush III (1984) Proc. Natl. Acad. Sci. USA 81, 5310–5314.
15. J. Sleep, C. Hermann, T. Barman, and F. Travers (1994) Biochemistry, 33, 6038–6042.
16. S. Lutsenko and J. H. Kaplan (1995) Biochemistry 34, 15607–15613.
17. J. H. Kaplan (1985) Annu. Rev. Physiol. 47, 534–544.
18. J. H. Kaplan and R. J. Hollis (1980) Nature 288, 587–589.
19. J. H. Kaplan (1982) J. Gen. Physiol. 80, 915–937.
20. D. Pascolini et al. (1988) Biophys. J. 54, 679–688.
21. K. Fendler, E. Grell, M. Haubs, and E. Bamberg (1985) EMBO J. 4, 3079–3085.
22. R. Borlinghaus, H.-J. Apell, and P. Lauger (1987) J. Membr. Biol. 97, 161–178.
23. E. Bamberg, H.-J. Butt, A. Eisenrauch, and K. Fendler (1993) Q. Rev. Biophys., 26, 1–25.
24. I. Wuddel and H.-J. Apell (1995) Biophys. J. 69, 909–921.
25. M. Stengelin, K. Fendler, and E. Bamberg (1993) J. Membr. Biol. 132, 211–227.
26. K. Fendler, S. Drose, K. Altendorf, and E. Bamberg (1996) Biochemistry, 35, 8009–8017.
27. A. F. Huxley and R. Niedergerke (1954) Nature 173, 971–973.
28. H. E. Huxley and J. Hanson (1954) Nature 173, 973–976.
29. Y. E. Goldman, M. G. Hibberd, J. A. McCray, and D. R. Trentham (1982) Nature 300, 701–705.
30. E. Homsher and N. C. Millar (1990) Annu. Rev. Physiol. 52, 875–896.
31. J. W. Tanner, D. D. Thomas, and Y. E. Goldman (1992) J. Mol. Biol. 223, 185–203.
32. C. L. Berger, F. C. Svensson, and D. D. Thomas (1989) Proc. Natl. Acad. Sci. USA 86, 8753–8757 .
33. K. J. V. Poole, G. Rapp, Y. Maeda, and R. S. Goody (1988) Adv. Exp. Med. Biol. 226, 391–404 .
34. K. Horiuti et al. (1994) J. Biochem. 115, 953–957.
35. A. P. Somlyo and A. V. Somlyo (1994) Nature 372, 231–236.
36. A. P. Somlyo and A. V. Somlyo (1990) Annu. Rev. Physiol. 52, 857–874.
37. B. Zimmerman et al. (1995) J. Biol. Chem. 270, 23966–23974.
38. A. J. Scheidig et al. (1995) J. Mol. Biol. 253, 132–150.
39. C. Raimbault et al. (1997) Eur. J. Biochem. 250, 773–782.
40. G. D. Housley, N. P. Raybould, and P. R. Thorne (1998) Hear. Res. 119, 1–13.
41. J. Hadju and L. N. Johnson (1990) Biochemistry 29, 1669–1678.
42. I. Schlichting et al. (1990) Nature 345, 309–314.
43. B. O''Rourke, P. H. Backx, and E. Marban (1992) Science 257, 245–248.
44. M. Higuchi, E. Muto, Y. Inoue, and T. Yanagida (1997) Proc. Natl. Acad. Sci. USA, 94, 4395–4400.
45. K. Lee, A. K. Dixon, T. C. Freeman, and P. J. Richardson (1998) J. Physiol. 510, 441–453.
46. T. Tani and S. Kamimura (1998) J. Exp. Biol. 201, 1493–1503.
47. M. N. Faddis and J. E. Brown (1992) J. Gen. Physiol. 100, 547–570.



|
|
|
|
4 أسباب تجعلك تضيف الزنجبيل إلى طعامك.. تعرف عليها
|
|
|
|
|
|
|
أكبر محطة للطاقة الكهرومائية في بريطانيا تستعد للانطلاق
|
|
|
|
|
|
|
العتبة العباسية المقدسة تبحث مع العتبة الحسينية المقدسة التنسيق المشترك لإقامة حفل تخرج طلبة الجامعات
|
|
|