


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
أقرأ أيضاً
التاريخ: 4-10-2020
التاريخ: 10-7-2018
التاريخ: 8-7-2019
التاريخ: 21-1-2022
|
Polymerization of an alkene by acidic reagents can be formulated by a mechanism similar to the addition of hydrogen halides to alkene linkages. First, a proton from a suitable acid adds to an alkene to yield a carbocation. Then, in the absence of any other reasonably strong nucleophilic reagent, another alkene molecule donates an electron pair and forms a longer-chain cation. Continuation of this process can lead to a high-molecular-weight cation. Termination can occur by loss of a proton. The following equations represent the overall reaction sequence:
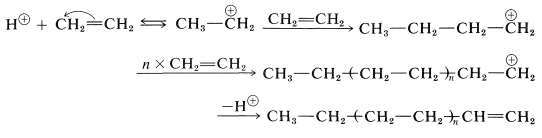
Ethene does not polymerize by the cationic mechanism because it does not have sufficiently electron-donating groups to permit easy formation of the intermediate growing-chain cation. 2-Methylpropene has electron-donating alkyl groups and polymerizes much more easily than ethene by this type of mechanism. The usual catalysts for cationic polymerization of 2-methylpropene are sulfuric acid, hydrogen fluoride, or a complex of boron trifluoride and water. Under nearly anhydrous conditions a very long chain polymer called polyisobutylene is formed.
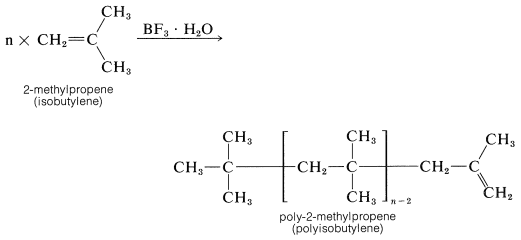
Polyisobutylene fractions of particular molecular weights are very tacky and are used as adhesives for pressure-sealing tapes.
In the presence of 60% sulfuric acid, 2-methylpropene is not converted to a long-chain polymer, but to a mixture of eight-carbon alkenes. The mechanism is like that of the polymerization of 2-methylpropene under nearly anhydrous conditions, except that chain termination occurs after only one 2-methylpropene molecule has been added:
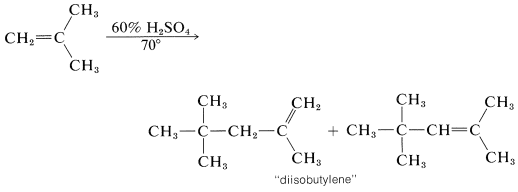
The short chain length is due to the high water concentration; the intermediate carbocation loses a proton to water before it can react with another alkene molecule.
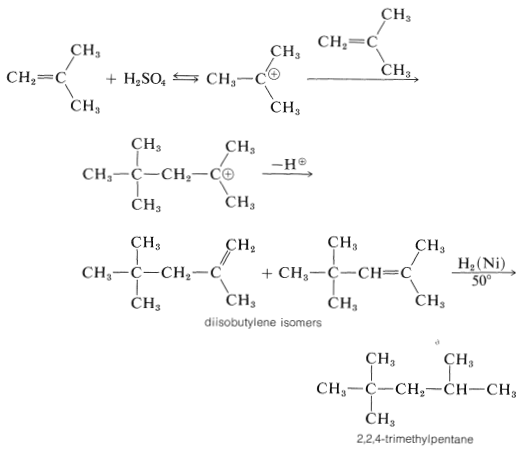
The proton can be lost in two different ways, and a mixture of alkene isomers is obtained. The alkene mixture is known as "diisobutylene" and has a number of commercial uses. Hydrogenation yields 2,2,4-trimethylpentane (often erroneously called "isooctane"), which is used as the standard "100 antiknock rating" fuel for internal-combustion gasoline engines:



|
|
|
|
للعاملين في الليل.. حيلة صحية تجنبكم خطر هذا النوع من العمل
|
|
|
|
|
|
|
"ناسا" تحتفي برائد الفضاء السوفياتي يوري غاغارين
|
|
|
|
|
|
|
ملاكات العتبة العباسية المقدسة تُنهي أعمال غسل حرم مرقد أبي الفضل العباس (عليه السلام) وفرشه
|
|
|