
Addition of Proton Acids to alkenes
 المؤلف:
John D. Roberts and Marjorie C. Caserio
المؤلف:
John D. Roberts and Marjorie C. Caserio
 المصدر:
Basic Principles of Organic Chemistry : LibreTexts project
المصدر:
Basic Principles of Organic Chemistry : LibreTexts project
 الجزء والصفحة:
........
الجزء والصفحة:
........
 14-1-2022
14-1-2022
 2578
2578
Addition of Proton Acids to alkenes
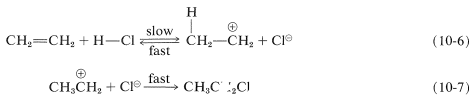
We have seen that electrophiles can react with alkenes to form carbon-halogen bonds by donating positive halogen, Br⊕, Cl⊕, or I⊕. Likewise, carbon-hydrogen bonds can be formed by appropriately strong proton donors, which, of course, are typically strong proton acids. These acids are more effective in the absence of large amounts of water because water can compete with the alkene as a proton acceptor. Hydrogen chloride addition to ethene occurs by way of a proton-transfer step to give the ethyl cation and a chloride ion (Equation 10-6) followed by a step in which the nucleophilic chloride ion combines with the ethyl cation (Equation 10-7):
All of the hydrogen halides HF, HCl, HBr, and HI) will add to alkenes. Addition of hydrogen fluoride, while facile, is easily reversible. However, a solution of 70% anhydrous hydrogen fluoride and 30% of the weak organic base, pyridine, which is about 1/10,000 times as strong as ammonia, works better, and with cyclohexene gives fluorocyclohexane. With hydrogen iodide, care must be taken to prevent I2 addition products resulting from iodine formed by oxidation reactions such as
4HI+O2→2I2+2H2O (10.4.1)
With hydrogen bromide, radical-chain addition may intervene unless the reaction conditions are controlled carefully.
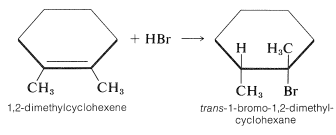
The stereochemistry of addition depends largely on the structure of the alkene, but for simple alkenes and cycloalkenes, addition occurs predominantly in an antarafacial manner. For example, hydrogen bromide reacts with 1,2-dimethylcyclohexene to give the antarafacial addition product:
 الاكثر قراءة في الهايدروكاربونات
الاكثر قراءة في الهايدروكاربونات
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


