
Selectivity in Alkane Halogenation
 المؤلف:
John D. Roberts and Marjorie C. Caserio
المؤلف:
John D. Roberts and Marjorie C. Caserio
 المصدر:
Basic Principles of Organic Chemistry : LibreTexts project
المصدر:
Basic Principles of Organic Chemistry : LibreTexts project
 الجزء والصفحة:
........
الجزء والصفحة:
........
 25-12-2021
25-12-2021
 2857
2857
Selectivity in Alkane Halogenation
For propane and higher hydrocarbons for which more than one monosubstitution product is generally possible, difficult separation problems bay arise when a particular product is desired. For example, the chlorination of 2-methylbutane 3 at 300o gives all four possible monosubstitution products, 4, 5, 6, and 7:
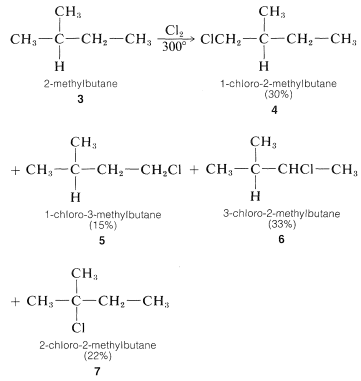
On a purely statistical basis, we may expect the ratio of products from 3 to correlate with the number of available hydrogens at the various positions of substitution. That is, 4, 5, 6, and 7 would be formed in the ratio 6:3:2:1 (50%:25%:17%:8%). However, as can be seen from Table 4-6, the strengths of hydrogen bonds to primary, secondary, and tertiary carbons are not the same and, from the argument we would expect the weaker C−H bonds to be preferentially attacked by Cl⋅. The proportion of 7 formed is about three times that expected on a statistical basis which is in accord with our expectation that the tertiary C−H bond of 2-methylbutane should be the weakest of the C−H bonds.
The factors governing selectivity in halogenation of alkanes follow:
1. The rates at which the various C−H bonds of 2-methylbutane are broken by attack of chlorine atoms approach 1:1:1 as the temperature is raised above 300o300o. At higher temperatures both chlorine atoms and hydrocarbons become more reactive because of increases in their thermal energies. Ultimately, temperatures are attained where a chlorine atom essentially removes the first hydrogen with which it collides regardless of position on the hydrocarbon chain. In such circumstances, the composition of monochlorination products will correspond to that expected from simple statistics.
2. Bromine atoms are far more selective than chlorine atoms. This is not unexpected because 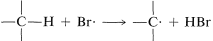 is endothermic, whereas corresponding reactions with a chlorine atoms usually are exothermic (data from Table 4-6). Bromine removes only those hydrogens that are relatively weakly bonded to a carbon atom.
is endothermic, whereas corresponding reactions with a chlorine atoms usually are exothermic (data from Table 4-6). Bromine removes only those hydrogens that are relatively weakly bonded to a carbon atom.

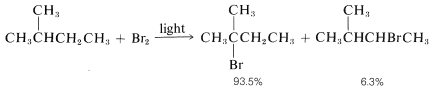
As predicted, attack of Br⋅Br⋅ on 2-methylbutane leads mostly to 2-bromo-2-methylbutane, some secondary bromide, and essentially no primary bromides:
3. The selectivity of chlorination reactions carried on in solution is increased markedly in the presence of benzene or alkyl-substituted benzenes because benzene and other arenes form loose complexes with chlorine atoms. This substantially cuts down chlorine-atom reactivity, thereby making the chlorine atoms behave more like bromine atoms.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


