
Larger Cycloalkanes
 المؤلف:
LibreTexts Project
المؤلف:
LibreTexts Project
 المصدر:
................
المصدر:
................
 الجزء والصفحة:
.................
الجزء والصفحة:
.................
 26-1-2020
26-1-2020
 1787
1787
Larger Cycloalkanes
The Baeyer strain theory suggested that the larger cycloalkanes ring are difficult to synthesize because of angle strain associated with planar rings, as calculated in Table 12-3. We now know that, except for cyclopropane, none of the cycloalkanes have planar carbon rings and that the higher cycloalkanes have normal or nearly normal bond angles. The reason that the higher cycloalkanes are generally difficult to synthesize from open-chain compounds is not so much angle strain, as Baeyer hypothesized, but the low probability of having reactive groups on the two fairly remote ends of a long hydrocarbon chain come together to effect cyclization. Usually, coupling of reactive groups on the ends of different molecules occurs in preference to cyclization, unless the reactions are carried out in very dilute solutions. This is called the high-dilution technique for achieving ring formation when the ring-forming reaction has to compete with rapid inter-molecular reactions.
With regard to conformations of the larger cycloalkanes, we first note that the chair form of cyclohexane is a “perfect” conformation for a cycloalkane. The C−C−C
bond angles are close to their normal values, all the adjacent hydrogens are staggered with respect to one another, and the 1,3-axial hydrogens are not close enough together to experience nonbonded repulsions. About the only qualification one could put on the ideality of the chair form is that the trans conformation of butane is somewhat more stable than the gauche conformation (Section 5-2), and that all of the C−C−C−C segments of cyclohexane have the gauche arrangement. Arguing from this, J. Dale6 has suggested that large cycloalkane rings would tend to have trans C−C−C−C segments to the degree possible and, indeed, cyclotetradecane seems to be most stable in a rectangular conformation with trans C−C−C−C bond segments (Figure 1.1). This conformation has a number of possible substituent positions, but because only single isomers of monosubstituted cyclotetradecanes have been isolated, rapid equilibration of the various conformational isomers must occur. Other evidence indicates that the barrier to interconversion of these conformations is about 7kcal mol−1.
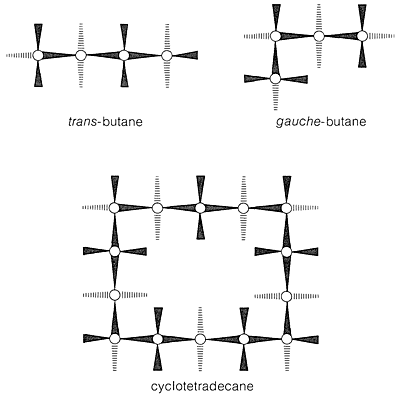
Figure 1.1 Favored conformation of cyclotetradecane as proposed by Dale. For comparison, the trans and gauche forms of butane are shown by the same convention. (The convention implies that the wedged lines are C−C or C−H bonds projecting out of the plane of the paper, with the wide end closest to you, and the broken lines are C−H bonds projecting behind the plane of the paper. The result is an “aerial” view of the molecule in the most stable staggered conformation.)
With the cycloalkanes having 7 to 10 carbons, there are problems in trying to make either trans or gauche C−C−C−C segments, because the sizes of these rings do not allow the proper bond angles or torsional angles, or else there are more or less serious nonbonded repulsions. Consequently each of these rings assumes a compromise conformation with some eclipsing, some nonbonded repulsions, and some angle distortions. Brief comments on some of these conformations follow. It will be useful to use molecular models to see the interactions involved.
 الاكثر قراءة في الهايدروكاربونات
الاكثر قراءة في الهايدروكاربونات
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


