
Carbonyl Condensations with Enamines - The Stork Reaction
 المؤلف:
..................
المؤلف:
..................
 المصدر:
LibreTexts Project
المصدر:
LibreTexts Project
 الجزء والصفحة:
.................
الجزء والصفحة:
.................
 26-11-2019
26-11-2019
 2091
2091
Carbonyl Condensations with Enamines - The Stork Reaction
As previously seen, aldehydes and ketones react with 2o amines to reversibly form enamines.
Example
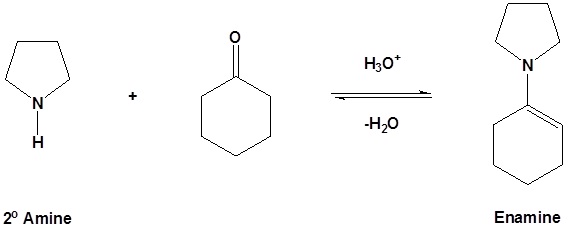
Reversible
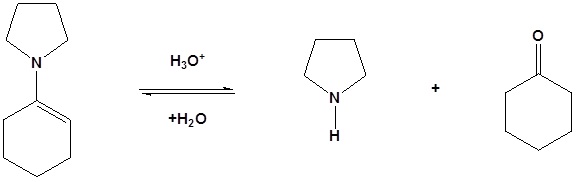
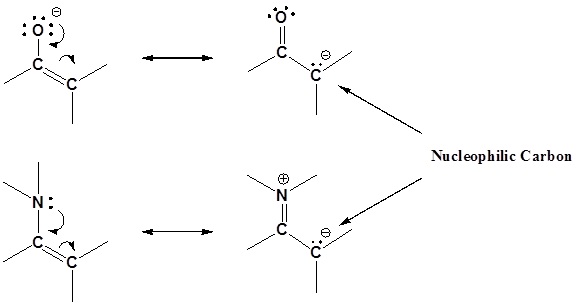
Enamines act as nucleophiles in a fashion similar to enolates. Because of this enamines can be used as synthetic equivalents as enolates in many reactions. This process requires a three steps: 1) Formation of the enamine, 2) Reaction with an eletrophile to form an iminium salt, 3) Hydrolysis of the iminium salt to reform the aldehyde or ketone. Some of the advantages of using an enamine over and enolate are enamines are neutral, easier to prepare, and usually prevent the overreaction problems plagued by enolates. These reactions are generally known as the Stork enamine reaction after Gilbert Stork of Columbia University who originated the work.
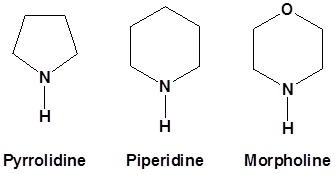
Typically we use the following 2o amines for enamine reactions
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


