

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Post translational Modification
المؤلف:
William Reusch
المصدر:
Virtual Textbook of Organic Chemistry
الجزء والصفحة:
............
27-8-2018
3510
Post-translational Modification
Once a peptide or protein has been synthesized and released from the ribosome it often undergoes further chemical transformation. This post-translational modification may involve the attachment of other moieties such as acyl groups, alkyl groups, phosphates, sulfates, lipids and carbohydrates. Functional changes such as dehydration, amidation, hydrolysis and oxidation (e.g. disulfide bond formation) are also common. In this manner the limited array of twenty amino acids designated by the codons may be expanded in a variety of ways to enable proper functioning of the resulting protein. Since these post-translational reactions are generally catalyzed by enzymes, it may be said: Virtually every molecule in a cell is made by the ribosome or by enzymes made by the ribosome.
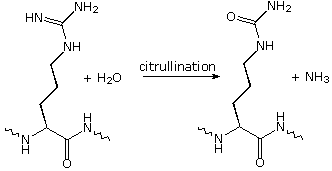
Modifications, like phosphorylation and citrullination, are part of common mechanisms for controlling the behavior of a protein. As shown on the left below, citrullination is the post-translational modification of the amino acid arginine into the amino acid citrulline. Arginine is positively charged at a neutral pH, whereas citrulline is uncharged, so this change increases the hydrophobicity of a protein. Phosphorylation of serine, threonine or tyrosine residues renders them more hydrophilic, but such changes are usually transient, serving to regulate the biological activity of the protein. Other important functional changes include iodination of tyrosine residues in the peptide thyroglobulin by action of the enzyme thyroperoxidase. The monoiodotyrosine and diiodotyrosine formed in this manner are then linked to form the thyroid hormones T3 and T4, shown on the right below.
 |
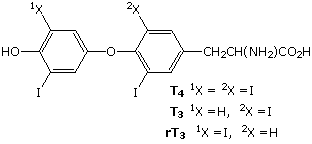 |
|---|
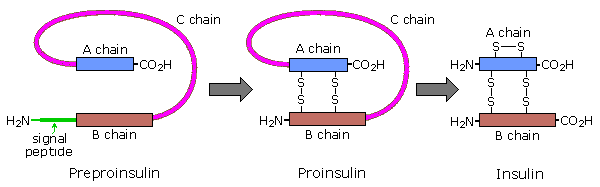
Amino acids may be enzymatically removed from the amino end of the protein. Because the "start" codon on mRNA codes for the amino acid methionine, this amino acid is usually removed from the resulting protein during post-translational modification. Peptide chains may also be cut in the middle to form shorter strands. Thus, insulin is initially synthesized as a 105 residue preprotein. The 24-amino acid signal peptide is removed, yielding a proinsulin peptide. This folds and forms disulfide bonds between cysteines 7 and 67 and between 19 and 80. Such dimeric cysteines, joined by a disulfide bond, are named cystine. A protease then cleaves the peptide at arg31 and arg60, with loss of the 32-60 sequence (chain C). Removal of arg 31 yields mature insulin, with the A and B chains held together by disulfide bonds and a third cystine moiety in chain A. The following cartoon illustrates this chain of events.
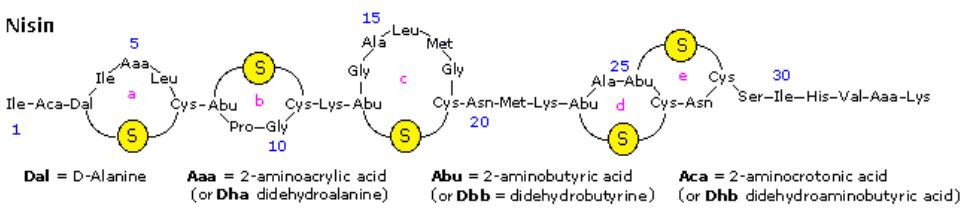
Nisin is a polypeptide (34 amino acids) made by the bacterium Lactococcus lactis. Nisin kills gram positive bacteria by binding to their membranes and targeting lipid II, an essential precursor of cell wall synthesis. Such antimicrobial peptides are a growing family of compounds which have received the name lantibiotics due to the presence of lanthionine, a nonproteinogenic amino acid with the chemical formula HO2C-CH(NH2)-CH2-S-CH2-CH(NH2)-CO2H. Lanthionine is composed of two alanine residues that are crosslinked on their β-carbon atoms by a thioether linkage (i.e. it is the monosulfide analog of the disulfide cystine). Lantibiotics are unique in that they are ribosomally synthesized as prepeptides, followed by post-translational processing of a number of amino acids (e.g. serine, threonine and cysteine) into dehydro residues and thioether crossbridges. Nisin is the only bacteriocin that is accepted as a food preservative. Several nisin subtypes that differ in amino acid composition and biological activity are known.
The bacterial cell wall is a cross-linked glycan polymer that surrounds bacterial cells, dictates their cell shape, and prevents them from breaking due to environmental changes in osmotic pressure. This wall consists mainly of peptidoglycan or murein, a three-dimensional polymer of sugars and amino acids located on the exterior of the cytoplasmic membrane.
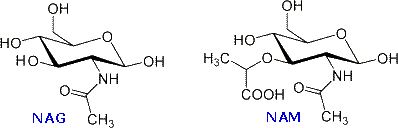
The monomer units are composed of two amino sugars, N-acetylglucosamine (NAG) and N-acetylmuramic acid (NAM), shown on the right. Transglycosidase enzymes join these units by glycoside bonds, and they are further interlinked to each other via peptide cross-links between the pentapeptide moieties that are attached to the NAM residues. Peptidoglycan subunits are assembled on the cytoplasmic side of the bacterial membrane from a polyisoprenoid anchor. Lipid II, a membrane-anchored cell-wall precursor that is essential for bacterial cell-wall biosynthesis, is one of the key components in the synthesis of peptidoglycan. Peptidoglycan synthesis via polymerization of Lipid II is illustrated in the following diagram. Cross-linking of the peptide side chains is then effected by transpeptidase enzymes. A model of Lipid II complexed with nisin may be examined as part of the previous Jmol display.
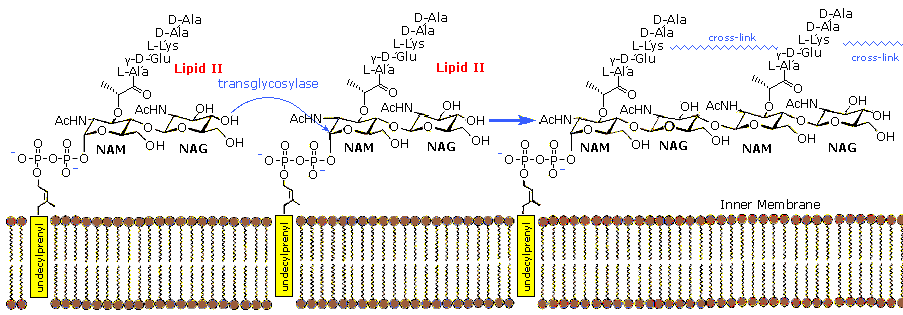
In order for bacteria to divide by binary fission and increase their size following division, links in the peptidoglycan must be broken, new peptidoglycan monomers must be inserted, and the peptide cross links must be resealed. Transglycosidase enzymes catalyze the formation of glycosidic bonds between the NAM and NAG of the peptidoglycan monomers and the NAG and NAM of the existing peptidoglycan. Finally, transpeptidase enzymes reform the peptide cross-links between the rows and layers of peptidoglycan making the wall strong. Many antibiotic drugs, including penicillin, target the chemistry of cell wall formation. The effectiveness of choosing Lipid II for an antibacterial strategy is highlighted by the fact that it is the target for at least four different classes of antibiotic, including the clinically important glycopeptide antibiotic vancomycin. The growing problem of bacterial resistance to many current drugs, including vancomycin, has led to increasing interest in the therapeutic potential of other classes of compound that target Lipid II. Lantibiotics such as nisin are part of this interest.
 الاكثر قراءة في الاحماض النووية
الاكثر قراءة في الاحماض النووية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















