

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Conformations of Disubstituted Cyclohexanes
المؤلف:
John McMurry
المصدر:
Organic Chemistry
الجزء والصفحة:
9th ed - p107
19-9-2016
3626
Conformations of Disubstituted Cyclohexanes
Monosubstituted cyclohexanes are always more stable with their substituent in an equatorial position, but the situation with disubstituted cyclohexanes is more complex because the steric effects of both substituents must be taken into account. All steric interactions for both possible chair conformations must be analyzed before deciding which conformation is favored. Let’s look at 1,2-dimethylcyclohexane as an example. There are two isomers, cis-1,2-dimethylcyclohexane and trans-1,2-dimethylcyclohexane, which must be considered separately. In the cis isomer, both methyl groups are on the same face of the ring and the compound can exist in either of the two chair conformations shown in Figure 1-1. (It may be easier for you to see whether a compound is cis- or trans-disubstituted by first drawing the ring as a flat representation and then converting it to a chair conformation.)
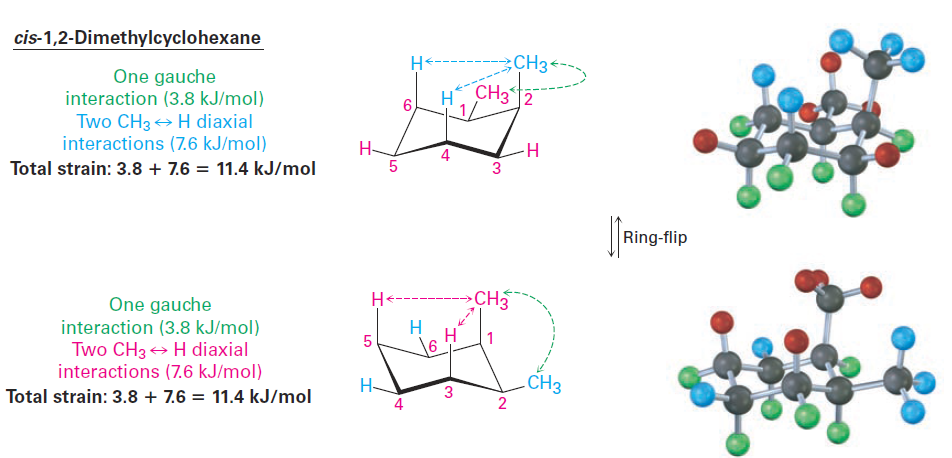
Figure 1-1 Conformations of cis-1,2-dimethylcyclohexane. The two chair conformations are equal in energy because each has one axial methyl group and one equatorial methyl group.
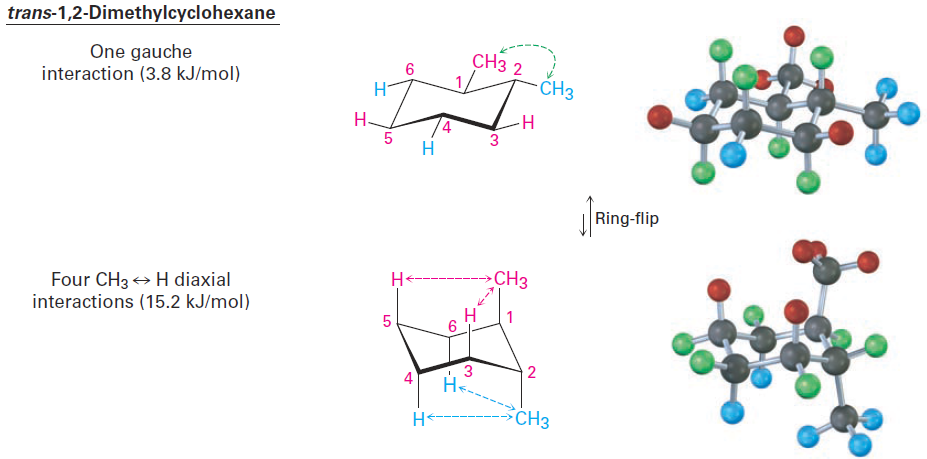
Figure 1-2 Conformations of trans-1,2-dimethylcyclohexane. The conformation with both methyl groups equatorial (top) is favored by 11.4 kJ/mol (2.7 kcal/mol) over the conformation with both methyl groups axial (bottom).
Both chair conformations of cis-1,2-dimethylcyclohexane have one axial methyl group and one equatorial methyl group. The top conformation in Figure 1-1 has an axial methyl group at C2, which has 1,3-diaxial interactions with hydrogens on C4 and C6. The ring-flipped conformation has an axial methyl group at C1, which has 1,3-diaxial interactions with hydrogens on C3 and C5. In addition, both conformations have gauche butane interactions between the two methyl groups. The two conformations are equal in energy, with a total steric strain of 3 x 3.8 kJ/mol = 11.4 kJ/mol (2.7 kcal/mol).
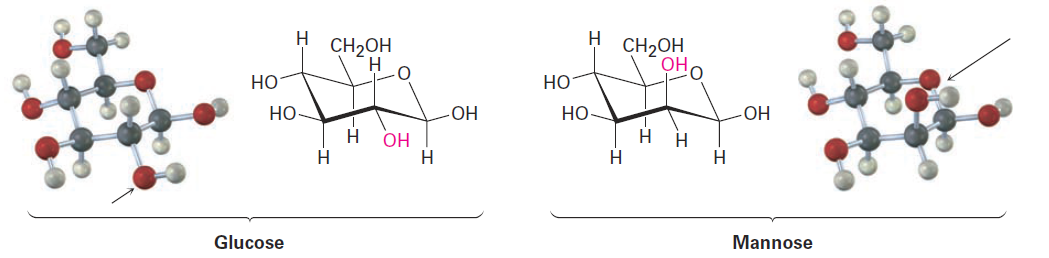
In trans-1,2-dimethylcyclohexane, the two methyl groups are on opposite faces of the ring and the compound can exist in either of the two chair conformations shown in Figure 1-2. The situation here is quite different from that of the cis isomer. The top conformation in Figure 1-2 has both methyl groups equatorial with only a gauche butane interaction between them (3.8 kJ/mol) but no 1,3-diaxial interactions. The ring-flipped conformation, however, has both methyl groups axial. The axial methyl group at C1 interacts with axial hydrogens at C3 and C5, and the axial methyl group at C2 interacts with axial hydrogens at C4 and C6. These four 1,3-diaxial interactions produce a steric strain of 4 x 3.8 kJ/mol = 15.2 kJ/mol and make the diaxial conformation 15.2 - 3.8 = 11.4 kJ/mol less favorable than the diequatorial conformation. We therefore predict that trans-1,2-dimethylcyclohexane will exist almost exclusively in the diequatorial conformation. The same kind of conformational analysis just carried out for cis- and trans-1,2-dimethylcyclohexane can be done for any substituted cyclohexane, such as cis-1-tert-butyl-4-chlorocyclohexane. As you might imagine, though, the situation becomes more complex as the number of substituents increases. For instance, compare glucose with mannose, a carbohydrate present in seaweed. Which do you think is more strained? In glucose, all substituents on the six-membered ring are equatorial, while in mannose, one of the -OH groups is axial, making it more strained.
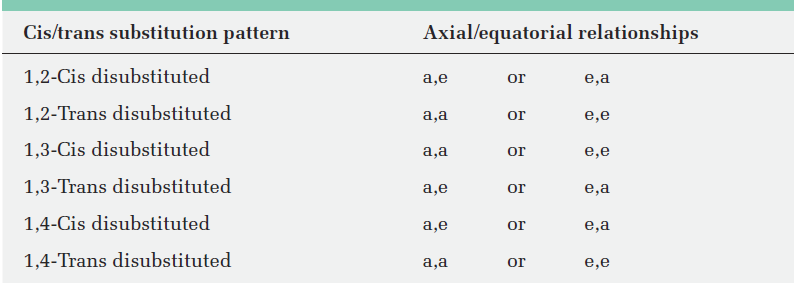
Table 1-1 Axial and Equatorial Relationships in Cis- and Trans-Disubstituted Cyclohexanes
 الاكثر قراءة في الهايدروكاربونات
الاكثر قراءة في الهايدروكاربونات
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















