

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Oxidation of Alkenes: Epoxidation and Hydroxylation
المؤلف:
John McMurry
المصدر:
Organic Chemistry
الجزء والصفحة:
9th ed - p239
21-8-2016
4663
Oxidation of Alkenes: Epoxidation and Hydroxylation
Like the word reduction used in the previous section for the addition of hydrogen to a double bond, the word oxidation has a slightly different meaning in organic chemistry from what you might have previously learned. In general chemistry, an oxidation is defined as the loss of one or more electrons by an atom. In organic chemistry, however, an oxidation is a reaction that results in a loss of electron density for carbon, caused either by bond formation between carbon and a more electronegative atom—usually oxygen, nitrogen, or a halogen or by bond-breaking between carbon and a less electronegative atom usually hydrogen. Note that an oxidation often adds oxygen, while a reduction often adds hydrogen.
Oxidation Decreases electron density on carbon by:
– forming one of these: C-O C-N C-X
– or breaking this: C-H
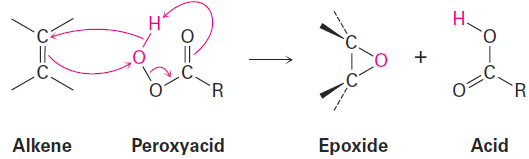
In the laboratory, alkenes are oxidized to give epoxides on treatment with a peroxyacid, RCO3H, such as meta-chloroperoxybenzoic acid. An epoxide, also called an oxirane, is a cyclic ether with an oxygen atom in a three-membered ring. For example:

Peroxyacids transfer an oxygen atom to the alkene with syn stereochemistry both C - O bonds form on the same face of the double bond through a one-step mechanism without intermediates. The oxygen atom farthest from the carbonyl group is the one transferred.
Another method for the synthesis of epoxides involves the use of halohydrins, prepared by electrophilic addition of HO - X to alkenes. When a halohydrin is treated with base, HX is eliminated and an epoxide is produced.

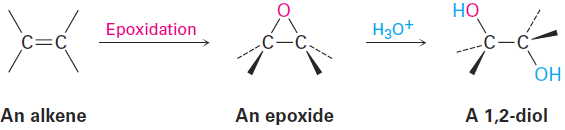
Epoxides undergo an acid-catalyzed ring-opening reaction with water (a hydrolysis) to give the corresponding 1,2-dialcohol, or diol, also called a glycol. Thus, the net result of the two-step alkene epoxidation/hydrolysis is hydroxylation the addition of an - OH group to each of the two doublebond carbons. In fact, approximately 18 million metric tons of ethylene glycol, HOCH2CH2OH, most of it used for automobile antifreeze, are produced worldwide each year by the epoxidation of ethylene and subsequent hydrolysis.
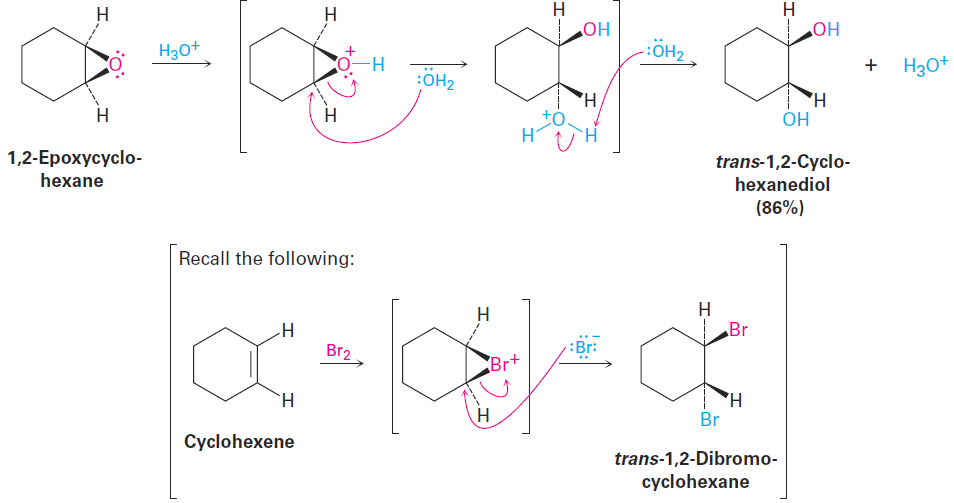
Acid-catalyzed epoxide opening begins with protonation of the epoxide to increase its reactivity, followed by nucleophilic addition of water. This nucleophilic addition is analogous to the final step of alkene bromination, in which a cyclic bromonium ion is opened by a nucleophile. That is, a trans-1,2-diol results when an epoxycycloalkane is opened by aqueous acid, just as a trans-1,2-dibromide results when a cycloalkene is brominated.
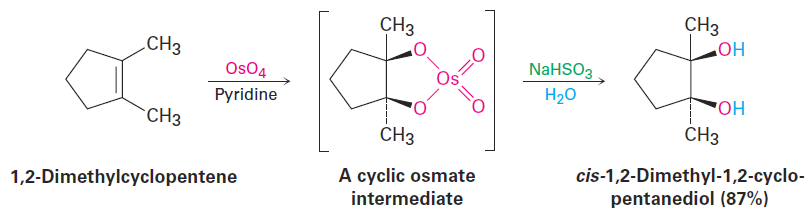
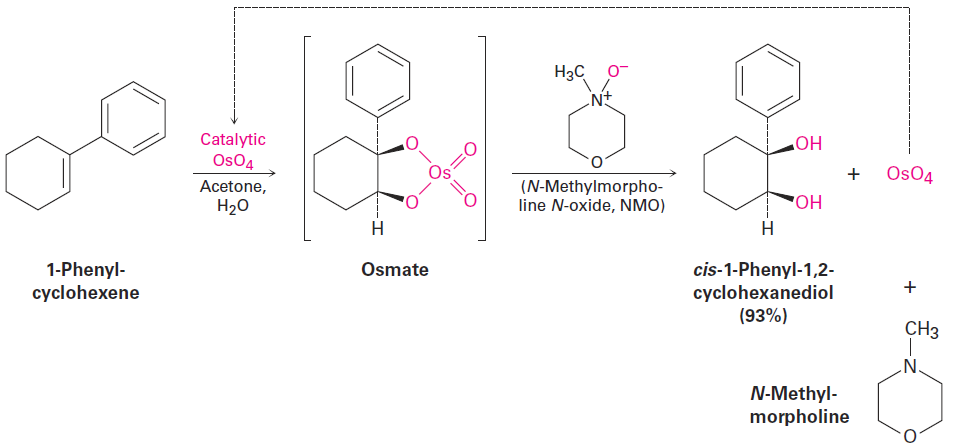
Hydroxylation can be carried out directly (without going through an intermediate epoxide) by treating an alkene with osmium tetroxide, OsO4. The reaction occurs with syn stereochemistry and does not involve a carbocation intermediate. Instead, it takes place through an intermediate cyclic osmate, which is formed in a single step by addition of OsO4 to the alkene. This cyclic osmate is then cleaved using aqueous sodium bisulfite, NaHSO3.
Because OsO4 is both very expensive and very toxic, the reaction is usually carried out using only a small, catalytic amount of OsO4 in the presence of a stoichiometric amount of a safe and inexpensive co-oxidant such as N-methylmorpholine N-oxide, abbreviated NMO. The initially formed osmate intermediate reacts rapidly with NMO to yield the product diol plus N-methylmorpholine and reoxidized OsO4, which reacts with more alkene in a catalytic cycle.
 الاكثر قراءة في الهايدروكاربونات
الاكثر قراءة في الهايدروكاربونات
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















