
The reliability of the equation
 المؤلف:
Peter Atkins، Julio de Paula
المؤلف:
Peter Atkins، Julio de Paula
 المصدر:
ATKINS PHYSICAL CHEMISTRY
المصدر:
ATKINS PHYSICAL CHEMISTRY
 الجزء والصفحة:
ص19-20
الجزء والصفحة:
ص19-20
 2025-10-30
2025-10-30
 37
37
The reliability of the equation
We now examine to what extent the van der Waals equation predicts the behaviour of real gases. It is too optimistic to expect a single, simple expression to be the true equation of state of all substances, and accurate work on gases must resort to the virial equation, use tabulated values of the coefficients at various temperatures, and analyse the systems numerically. The advantage of the van der Waals equation, however, is that it is analytical (that is, expressed symbolically) and allows us to draw some general conclusions about real gases. When the equation fails we must use one of the other equations of state that have been proposed (some are listed in Table 1.7), invent a new one, or go back to the virial equation. That having been said, we can begin to judge the reliability of the equation by com paring the isotherms it predicts with the experimental isotherms in Fig. 1.15. Some
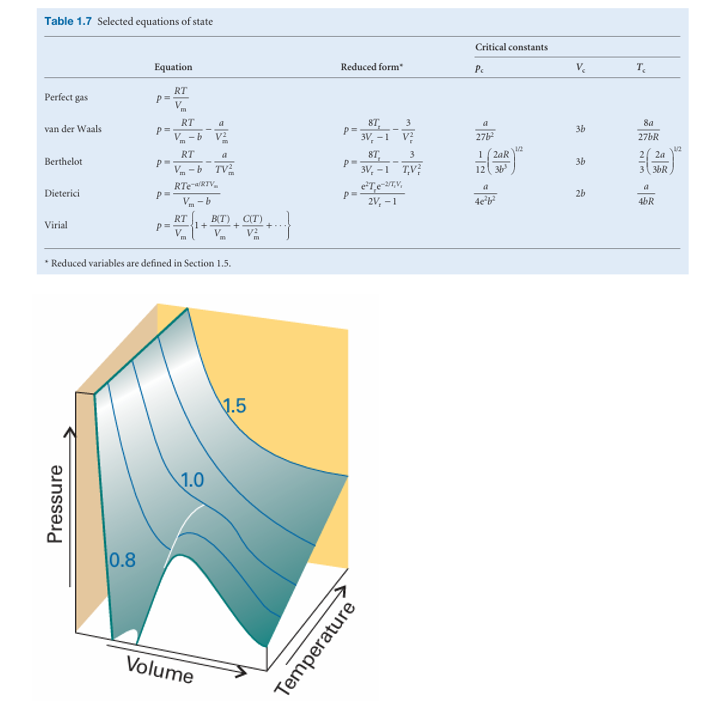
Fig. 1.17 The surface of possible states allowed by the van der Waals equation. Compare this surface with that shown in Fig. 1.8.
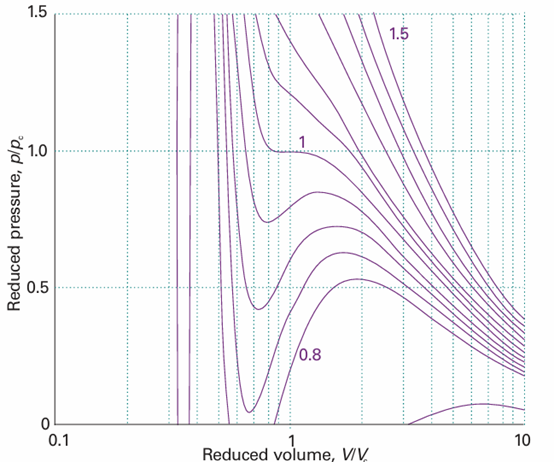
Fig. 1.18 Van der Waals isotherms at several values of T/Tc. Compare these curves with those in Fig. 1.15. The van der Waals loops are normally replaced by horizontal straight lines. The critical isotherm is the isotherm for T/Tc = 1. Exploration Calculate the molar volume of chlorine gas on the basis of the van der Waals equation of state at 250 K and 150 kPa and calculate the percentage difference from the value predicted by the perfect gas equation. calculated isotherms are shown in Figs. 1.17 and 1.18. Apart from the oscillations below the critical temperature, they do resemble experimental isotherms quite well. The oscillations, the van der Waals loops, are unrealistic because they suggest that under some conditions an increase of pressure results in an increase of volume. Therefore, they are replaced by horizontal lines drawn so the loops define equal areas above and below the lines: this procedure is called the Maxwell construction (3). The van der Waals coefficients, such as those in Table 1.7, are found by fitting the calcu lated curves to the experimental curves.
 الاكثر قراءة في مواضيع عامة في الكيمياء الفيزيائية
الاكثر قراءة في مواضيع عامة في الكيمياء الفيزيائية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


