

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Advances in inorganic framework chemistry
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
الجزء والصفحة:
ص636-639
2025-10-12
87
Advances in inorganic framework chemistry
Key point: Enormous structural diversity can be obtained from linked polyhedra and, with the use of templates, can lead to remarkable porous frameworks. The extensive development of aluminosilicates and zeolites has motivated synthetic inorganic chemists to seek similar structural types built from other tetrahedral and octahedral polyhedra for use in ion exchange, absorption, and catalysis. The use of different and larger polyhedra provides more flexibility in the framework topologies, and there is also the potential to incorporate d-metal ions with their associated properties of colour,
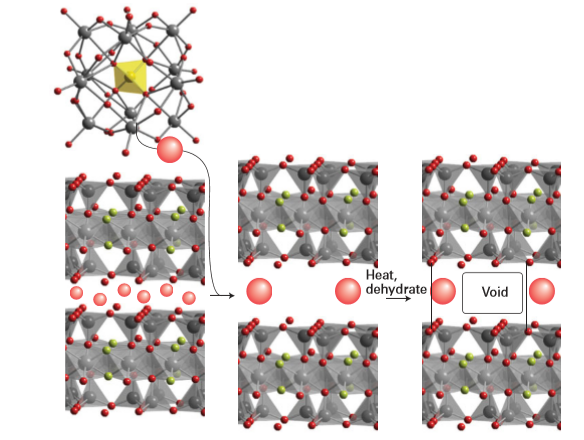
Figure 24.54 Schematic representation of pillaring of a clay by ion exchange of a simple monatomic interlayer cation with large polynuclear hydroxometallate followed by dehydration and cross-linking of the layers to form the cavities.
redox properties, and magnetism. Tetrahedral species that have been incorporated into such frameworks, as well as the aluminate, silicate, and phosphate groups mentioned previously, include ZnO4, AsO4, CoO4, GaO4, and GeO4. Octahedral units are mainly based on d metals and the heavier and larger metals from Groups 13 and 14. Other polyhedral units such as five-coordinate square pyramids also occur, but less commonly. Zeolite analogues are called zeotypes. Ring sizes larger than 12 tetrahedrally coordinated atoms were first seen in metallophosphate systems, and structures with 20 and 24 linked tetrahedra have been made. Aluminophosphates were the first microporous frameworks synthesized that contain polyhedra with coordination numbers greater than 4. Other socalled hypertetrahedral frameworks are now well established, such as the titanosilicate families (which have four-coordinate Si and five- and six-coordinate Ti sites) and a series of octahedral molecular sieves based on linked MnO6 units. Good examples of this structural family are the titanosilicate zeotypes built from SiO4 tetrahedra and various TiOn polyhedra with n 4–6. These compounds are made under the hydrothermal conditions similar to those used for synthesizing many zeolites but by using a source of Ti, such as TiCl4 or Ti(OC2H5)4, that hydrolyses under the basic conditions in the autoclave. Templates may also be used in such media and act as structuredirecting units giving rise to particular pore sizes and geometries. In a typical reaction the titanosilicate ETS-10 (Engelhard TitanoSilicate 10) is prepared by the reaction of TiCl4, sodium silicate, sodium hydroxide, and sometimes a template such as tetraethylammonium bromide, in a sealed polytetrafluorethylene-lined autoclave at between 150 and 230ºC. The number of titanosilicates continues to grow, but two materials, ETS-10 and Na2Ti2O3SiO4.2H2O, are worthy of further consideration here as specific examples of this type of material. ETS-10 is a microporous material built from TiO6 octahedra and SiO4 tetrahedra, with the TiO6 groups linked together in chains (Fig. 24.55a). The structure has 12-membered rings (that is, pores formed from 12-unit polyhedra) in all three directions.
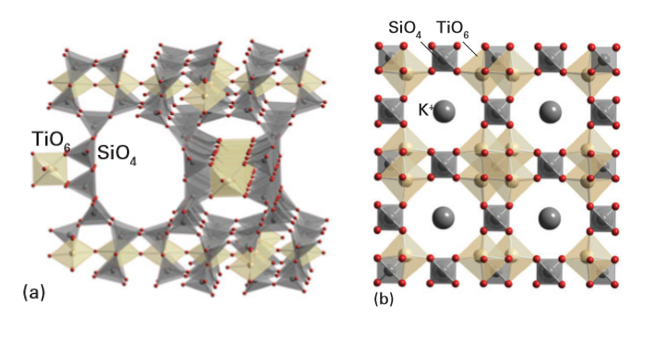
Figure 24.55 The structures of (a) the titanosilicate ETS-10, which is built from chains of TiO6 octahedra linked by SiO4 tetrahedra, and (b) K3H(TiO)4(SiO4)3.4H2O, a synthetic analogue of a natural mineral pharmacosiderite.
Na2Ti2O3SiO4.2H2O also has TiO6 octahedra, but in this structure these octahedra form clusters with four TiO6 units linked by the tetrahedral SiO4 units (as in Fig. 24.55a). This connectivity gives rise to large octagonal pores containing hydrated Naions. This compound, in common with several other titanosilicates such as K3H(TiO)4(SiO4)3·4H2O, a synthetic analogue of a natural mineral pharmacosiderite (Fig. 24.55b), shows excellent ion-exchange properties, particularly with large cations. These large ions replace Na+ with very high selectivity, so that, for example, Cs+ and Sr2+ ions may be extracted into the titanosilicate structure from their dilute solutions. This ability has led to the development of these materials for removal of radionuclides from nuclear waste, in which 137Cs and 90Sr are highly active.
The aim of much of the recent work on porous framework structures has been to incorporate d-metal ions. Materials that have been synthesized include antiferromagnetic porous iron (III) fluorophosphates with Néel temperatures in the range 10–40 K. These temperatures are relatively high for iron clusters linked by phosphate groups and indicate the presence of moderately strong magnetic interactions. Zeotypic cobalt (II), vanadium(V), titanium (IV), and nickel(II) phosphates have been prepared, including the material known as VSB-1 (Versailles Santa Barbara), which was the first microporous solid with 24-membered ring tunnels, and is simultaneously porous, magnetic, and an ion-exchange medium (Fig. 24.56). Two other rapidly growing areas of research into framework structures are materials based on sulfides and on the use of small organic molecules to link the metal ions, so giving rise to metal–organic frameworks (MOFs). Tetrahedral coordination is common in simple metal sulfide chemistry, as we saw in the discussion of ZnS in wurtzite and sphalerite (Section 3.9), and some compounds may be considered as containing linked fragments of these structures known as supertetrahedral clusters. For example, [N(CH3)4]4[Zn10S4(SPh)16] contains the isolated supertetrahedral unit Zn10S20 terminated by phenyl groups (Fig. 24.57). Metal–organic frameworks have structures that are based on bidentate or polydentate organic ligands lying between the metal atoms. Examples of simple ligands used to build these often-porous frameworks are CN-, nitriles, amines, and carboxylates. Many hundreds of these compounds have now been made, and include positively charged frameworks with balancing anions in the cavities, for instance Ag(4,4’-bpy) NO3, and electrically neutral frameworks, such as Zn2(1,3,5-benzenetricarboxylate) NO3.H2O. C2H5OH, from which the H2O molecules may be removed reversibly. The compounds have properties analogous to those of zeolites and of note is the framework of a chromium terephthalate (1,4-benzenedicarboxylate) which has pore diameters of about 3 nm and a specific internal surface area for N2 of over 5000 m2g-1 (Fig. 24.58).
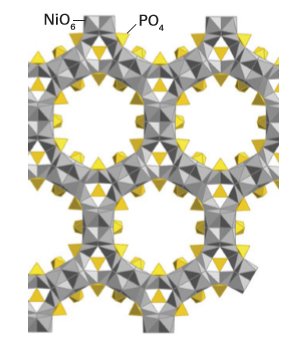
Figure 24.56 The framework structure of VSB-1 consists of linked NiO6 octahedra and PO4 tetrahedra. The main channel is bounded by 24 such units and has a diameter such that molecules up to 0.88 nm in diameter may pass through it.
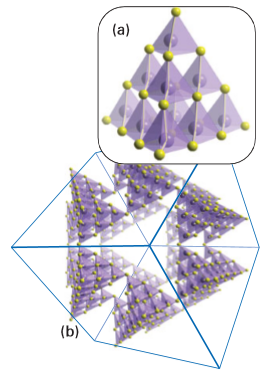
Figure 24.57 (a) An isolated supertetrahedral unit of stoichiometry Zn10S20 formed from individual ZnS4 tetrahedra. (b) These supertetrahedral units may be linked together asbuilding blocks to form large porous three-dimensional structures.
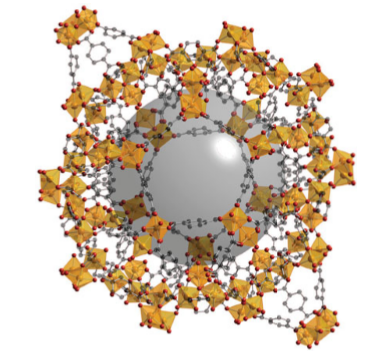
Figure 24.58 A metal–organic framework (MOF) formed from chromium terephthalate units showing CrO6 octahedra (gold) linked by the organic anions, The large central sphere shows the extent of the pore in this material, which can be filled with solvent or gas molecules.
These materials have potential applications in gas storage, for example of H2 and CO2. The very large pores allow large, complex molecules such as many pharmaceutically active compounds to be inserted into them and a further application is the use of these MOFs as drug delivery agents. Here an active compound, for example the painkiller ibuprofen, is adsorbed within the MOF pore from which it can then be released slowly, where required, in the human body.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















