

النبات

مواضيع عامة في علم النبات

الجذور - السيقان - الأوراق

النباتات الوعائية واللاوعائية

البذور (مغطاة البذور - عاريات البذور)

الطحالب

النباتات الطبية


الحيوان

مواضيع عامة في علم الحيوان

علم التشريح

التنوع الإحيائي

البايلوجيا الخلوية


الأحياء المجهرية

البكتيريا

الفطريات

الطفيليات

الفايروسات


علم الأمراض

الاورام

الامراض الوراثية

الامراض المناعية

الامراض المدارية

اضطرابات الدورة الدموية

مواضيع عامة في علم الامراض

الحشرات


التقانة الإحيائية

مواضيع عامة في التقانة الإحيائية


التقنية الحيوية المكروبية

التقنية الحيوية والميكروبات

الفعاليات الحيوية

وراثة الاحياء المجهرية

تصنيف الاحياء المجهرية

الاحياء المجهرية في الطبيعة

أيض الاجهاد

التقنية الحيوية والبيئة

التقنية الحيوية والطب

التقنية الحيوية والزراعة

التقنية الحيوية والصناعة

التقنية الحيوية والطاقة

البحار والطحالب الصغيرة

عزل البروتين

هندسة الجينات


التقنية الحياتية النانوية

مفاهيم التقنية الحيوية النانوية

التراكيب النانوية والمجاهر المستخدمة في رؤيتها

تصنيع وتخليق المواد النانوية

تطبيقات التقنية النانوية والحيوية النانوية

الرقائق والمتحسسات الحيوية

المصفوفات المجهرية وحاسوب الدنا

اللقاحات

البيئة والتلوث


علم الأجنة

اعضاء التكاثر وتشكل الاعراس

الاخصاب

التشطر

العصيبة وتشكل الجسيدات

تشكل اللواحق الجنينية

تكون المعيدة وظهور الطبقات الجنينية

مقدمة لعلم الاجنة


الأحياء الجزيئي

مواضيع عامة في الاحياء الجزيئي


علم وظائف الأعضاء


الغدد

مواضيع عامة في الغدد

الغدد الصم و هرموناتها

الجسم تحت السريري

الغدة النخامية

الغدة الكظرية

الغدة التناسلية

الغدة الدرقية والجار الدرقية

الغدة البنكرياسية

الغدة الصنوبرية

مواضيع عامة في علم وظائف الاعضاء

الخلية الحيوانية

الجهاز العصبي

أعضاء الحس

الجهاز العضلي

السوائل الجسمية

الجهاز الدوري والليمف

الجهاز التنفسي

الجهاز الهضمي

الجهاز البولي


المضادات الحيوية

مواضيع عامة في المضادات الحيوية

مضادات البكتيريا

مضادات الفطريات

مضادات الطفيليات

مضادات الفايروسات

علم الخلية

الوراثة

الأحياء العامة

المناعة

التحليلات المرضية

الكيمياء الحيوية

مواضيع متنوعة أخرى

الانزيمات
Laboratory Diagnosis of Haemophilus
المؤلف:
Patricia M. Tille, PhD, MLS(ASCP)
المصدر:
Bailey & Scotts Diagnostic Microbiology
الجزء والصفحة:
13th Edition , p404-407
2025-08-07
62
SPECIMEN COLLECTION AND TRANSPORT
Haemophilus spp. can be isolated from most clinical specimens. with emphasis on the following points. First, Haemophilus spp. are susceptible to drying and temperature extremes. Therefore, specimens suspected of containing these organisms should be inoculated to the appropriate media immediately. Specimens susceptible to contamination with normal flora such as a lower respiratory specimen should be collected by bronchioalveolar lavage. In cases of pneumonia or cerebrospinal fluid (CSF) infection or suspected infection of any other normally sterile body fluid, blood cultures should also be collected.
Second, the recovery of H. ducreyi from genital ulcers requires special processing. The ulcer should be cleaned with sterile gauze moistened with sterile saline. A cotton swab moistened with phosphate-buffered saline is then used to collect material from the base of the ulcer. To maximize the chance for recovering the organism, the swab must be plated to special selective media within 10 minutes of collection.
SPECIMEN PROCESSING
Other than the precautions required for the collection of H. ducreyi, no special considerations are required for specimen processing of Haemophilus spp.
DIRECT DETECTION METHODS
Direct Observation
Gram stain is generally used for the direct detection of Haemophilus in clinical material (Figure1). However, in some instances the acridine orange stain (AO) is used to detect smaller numbers of organisms that may be undetectable by gram staining.
Fig1. Gram stain of Haemophilus influenzae.
To increase the sensitivity of direct Gram stain examination of body fluid specimens, especially CSF, specimens may be centrifuged (2000 rpm for 10 minutes) and the smear is prepared from the pellet deposited in the bottom of the tube. Most laboratories are now equipped with a cytocentrifuge (10,000 × g for 10 minutes) used for concentration of specimens. This is highly recommended over traditional centrifugation for non-turbid specimens. This concentration step can increase the sensitivity of direct microscopic examination from five to tenfold. Moreover, cytocentrifugation of the specimen, in which clinical material is concentrated by centrifugation directly onto microscope slides, reportedly increases sensitivity of the Gram stain by as much as 100-fold.
Gram stains of the smears from clinical specimens must be examined carefully. Haemophilus spp. stain a pale pink and may be difficult to detect in the pink back ground of proteinaceous material often found in clinical specimens. Underdecolorization may result in misidentification of H. influenzae as either Streptococcus spp. or Listeria monocytogenes.
H. influenzae appears as pleomorphic coccobacilli or small rods, whereas the cells usually appear as long, slender rods. H. haemolyticus are small coccobacilli or short rods with occasional cells appearing as tangled filaments.
H. parainfluenzae produce either small pleomorphic rods or long filamentous forms, whereas H. parahaemolyticus usually are short to medium-length bacilli. Aggregatibacter aphrophilus is a very short bacillum but occasionally are seen as filamentous forms. H. ducreyi may be either slender or coccobacillary. Traditionally, H. ducreyi cells are described as appearing as “schools of fish.” However, this morphology is rarely seen in clinical specimens.
Table 1 presents Haemophilus influenzae and H. parainfluenzae biotypes.
Table1. Differentiation of Haemophilus influenzae and H. parainfluenzae Biotypes
Antigen Detection
Haemophilus influenzae type b capsular polysaccharide in clinical specimens, such as CSF and urine, can be detected directly using commercially available particle agglutination assays. Organisms in clinical infections are usually present at a sufficiently high concentration to be visualized by Gram stain. Therefore, most clinical laboratories no longer perform the latex test for the identification of Haemophilus spp. Latex tests are sensitive and specific for detection of H. influenzae type b, especially in patients treated with antimicrobial therapy prior to specimen collection. However, false positives have been reported in CSF and urine of patients who have recently immunized with the Hib vaccine.
Molecular Testing
Rapid screening procedures are very useful for patient therapy and evaluating outbreaks and have been developed for detection from CSF, plasma, serum, and whole blood. A PCR method for Haemophilus influenzae capsular types a and f has been developed. PCR product was amplified for the specific capsular type for which the primer was designed. PCR has its advantages over serotyping in that problems of cross-reaction and autoagglutination are gone. Detection from some clinical samples has been problematic based on the presence of small numbers of organisms in the sample increasing the need for large samples and concentration procedures.
Diagnosis of chancroid and the identification of H. ducreyi have been successfully completed using a variety of molecular targets. Amplification of the 16SrRNA, the rrs (16S)-rri (23S) spacer region, or the heat shock protein gene groEL has been used in molecular assays. Molecular methods have demonstrated improved sensitivity over traditional methods.
In addition to molecular methods for the identification, pulsed-field gel electrophoresis is considered the gold standard for typing Haemophilus isolates. Additional amplification methods such as repetitive-element sequence-based PCR, ribotyping, restriction fragment length polymorphism (RFLP), multilocus enzyme electrophoresis, and rapidly amplified polymorphic DNA (RAPD) have also been used.
CULTIVATION
Media of Choice
Haemophilus spp. typically grows on chocolate agar as smooth, flat or convex buff, or slightly yellow colonies. Chocolate agar provides hemin (X factor) and NAD (V factor), necessary for the growth of Haemophilus spp. Most strains will not grow on 5% sheep blood agar, which contains protoporphyrin IX but not NAD. Several bacterial species, including Staphylococcus aureus, produce NAD as a metabolic byproduct. Therefore, tiny colonies of Haemophilus spp. may be seen growing on sheep blood agar very close to colonies of bacteria capable of producing V factor; this is known as the satellite phenomenon (Figure 2). The satellite phenomenon has become important in this era of needing to rapidly identify potential agents of a bioterrorist attack. To examine an isolate for the satellite phenomenon, place a single streak of a hemolysin-producing strain of Staphylococcus spp. on a sheep blood agar plate that has been inoculated with a suspected Haemophilus spp. The Staphylococcus lyses the red blood cells adjacent to the streak line, releasing hemin (x factor) and NAD (v factor), providing the necessary components for growth of Haemophilus spp. Haemophilus spp. will grow adjacent to the streak line where the nutrients are available.
Fig2. Haemophilus influenzae satellite phenomenon (arrow) around colonies of Staphylococcus aureus.
A selective medium, such as horse blood–bacitracin agar, may be used for isolation of H. influenzae from respiratory secretions of patients with cystic fibrosis. This medium is designed to prevent overgrowth of H. influenzae by mucoid Pseudomonas aeruginosa. Haemophilus spp. are unable to grow on MacConkey agar.
H. ducreyi requires additional growth factors and special media for cultivation in the laboratory. Two types of media utilized within the laboratory include (1) Mueller-Hinton–based chocolate agar supplemented with 1% IsoVitaleX and 3 µg/mL vancomycin and (2) heart infusion–based agar supplemented with 10% fetal bovine serum and 3 µg/mL vancomycin. The vancomycin inhibits gram-positive colonizing organisms of the genital tract.
Haemophilus spp. will grow in commercial blood culture broth systems and in common nutrient broths such as thioglycollate and brain-heart infusion. However, the growth is often slower, produces weakly turbid sus pensions, and may not be readily visible in broth cultures. For this reason, blind subcultures to chocolate agar or examination of smears by AO or Gram stain have been used to enhance detection. Subcultures have not demonstrated a clinically significant effect on the isolation and detection of Haemophilus spp. from blood culture systems.
Rabbit or horse blood agars are commonly used for detecting hemolysis by hemolysin-producing strains of Haemophilus strains unable to grow on 5% sheep blood.
Incubation Conditions and Duration
Most strains of Haemophilus spp. are able to grow aerobically and anaerobically (facultative anaerobes). Growth is stimulated by 5% to 10% carbon dioxide (CO2). It is recommended that cultures be incubated in a candle extinction jar, CO2 pouch, or CO2 incubator. These organisms usually grow within 24 hours, but cultures are routinely held 72 hours before being discarded as negative. An exception is H. ducreyi, which may require as long as 7 days to grow.
Optimal growth of all Haemophilus spp., except H. ducreyi, occurs at 35° to 37° C. Cultures for H. ducreyi should be incubated at 33° C. In addition, H. ducreyi requires high humidity, which may be established by placing a sterile gauze pad moistened with sterile water inside the candle jar or CO2 pouch.
Colonial Appearance
Table 2 describes the colonial appearance and other distinguishing characteristics (e.g., odor and hemolysis) of each species.
Table2. Colonial Appearance and Characteristics
APPROACH TO IDENTIFICATION
Commercial identification systems for Haemophilus spp. are available. All of the systems incorporate several rapid enzymatic tests and generally work well for identifying these organisms.
Traditional identification criteria include hemolysis on horse or rabbit blood and the requirement for X and V factors for growth. To establish X and V factor requirements, disks impregnated with each factor are placed on unsupplemented media, usually Mueller-Hinton agar or trypticase soy agar, inoculated with a light suspension of the organism (see Figure 3). After overnight incubation at 35° C in ambient air, the plate is examined for growth around each disk. Many X factor–requiring organisms are able to carry over enough factor from the primary medium to give false-negative results (i.e., growth occurs at such a distance from the X disk as to falsely indicate that the organism does not require the X factor).
Fig3. Triple sugar iron agar. A, Acid slant/acid butt with gas, no H2S (A/A). B, Alkaline slant/acid butt, no gas, H2S-positive (K/A H2S+). C, Alkaline slant/alkaline butt, no gas, no H2S (K/K). D, Uninoculated tube.
The porphyrin test is another means for establishing an organism’s X-factor requirements and eliminates the potential problem of carryover. This test detects the presence of enzymes that convert δ-aminolevulinic acid (ALA) into porphyrins or protoporphyrins. The porphyrin test may be performed in broth, in agar, or on a disk.
Isolates from CSF or respiratory tract specimens that (1) are gram-negative rods or gram-negative coccobacilli, (2) grow on chocolate agar in CO2 but not blood agar or satellite around other colonies on blood agar, and (3) are porphyrin negative and nonhemolytic on rabbit or horse blood may be identified as H. influenzae. Haemophilus isolates may also be identified to species using rapid sugar fermentation tests; an abbreviated identification scheme for the X- and V-requiring organisms is shown in Table 3.
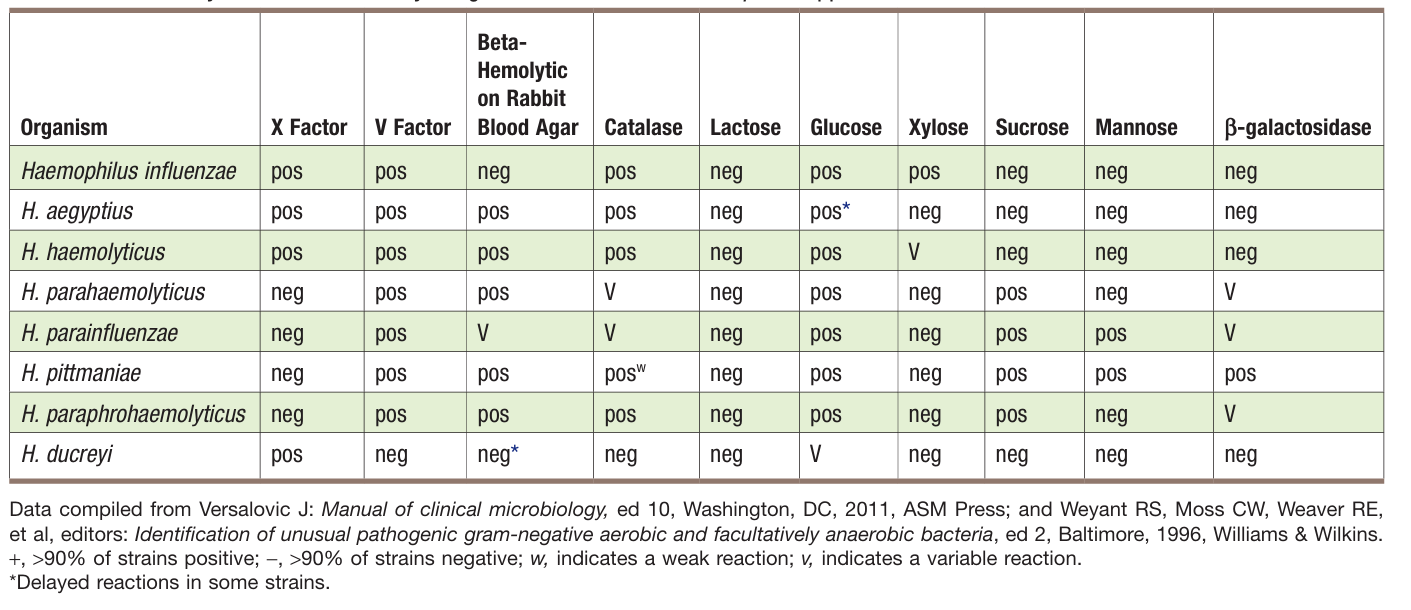
Table3. Key Biochemical and Physiologic Characteristics of Haemophilus spp.
SEROTYPING
Although serologic typing of H. influenzae may be used to establish an isolate as being any one of the six serotypes (i.e., a, b, c, d, e, and f), it is used primarily to identify type b strains. All H. influenzae from cases of invasive infections should be serotyped to determine whether or not H. influenzae type b is the cause of the infection. Testing can be performed using a slide agglutination test; a saline control without the reagent antibodies should always be tested simultaneously alongside the patient’s specimen in order to detect auto agglutination (i.e., the nonspecific agglutination of the test organism without homologous antiserum).
SERODIAGNOSIS
An enzyme-linked immunosorbent assay (ELISA) has been developed to detect antibodies to H. ducreyi. ELISA has been used to show seroconversion following Hib vaccination. None of these assays are commonly used for diagnostic purposes.
 الاكثر قراءة في البكتيريا
الاكثر قراءة في البكتيريا
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام) قسم الشؤون الفكرية يصدر مجموعة قصصية بعنوان (قلوب بلا مأوى)
قسم الشؤون الفكرية يصدر مجموعة قصصية بعنوان (قلوب بلا مأوى)


















