

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Nitrides Of the group 13 metals
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المصدر:
Inorganic Chemistry
الجزء والصفحة:
p 317
31-1-2018
1710
Nitrides Of the group 13 metals
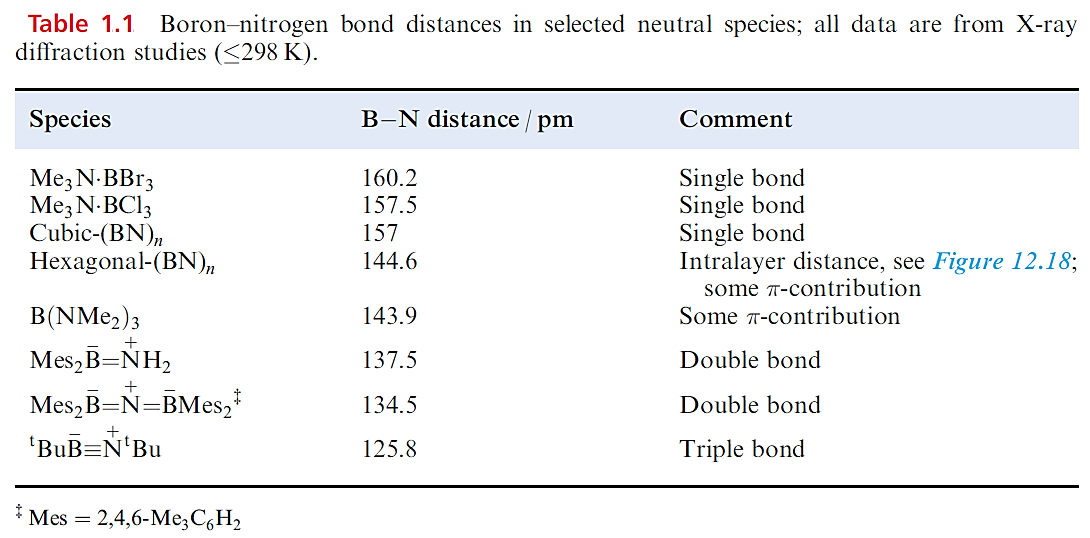
Boron nitride, BN, is a robust (sublimation point = 2603 K), chemically rather inert compound which is used as a ceramic material (e.g. in crucible manufacture). Preparative routes include the high-temperature reactions of borax with [NH4]+Cl, B2O3 with NH3, and B(OH)3 with [NH4]+Cl. High-purity boron nitride can be made by reacting NH3 with BF3 or BCl3. The common form of boron nitride has an ordered layer structure containing hexagonal rings (Figure 1.1). The layers are arranged so that a B atom in one layer lies directly over an N atom in the next, and so on. The B_N distances within a layer are much shorter than those between layers (Figure 1.1) and, in Table 1.1, it is compared with those in other B_N species. The B_N bonds are shorter than in adducts such as Me3N.BBr3 in which a single boron– nitrogen bond can be assigned, and imply the presence of π-bonding in BN resulting from overlap between orthogonal N 2p (occupied) and B 2p (vacant) orbitals. The interlayer distance of 330pm is consistent with van der Waals interactions, and boron nitride acts as a good lubricant, thus resembling graphite. Unlike graphite, BN is white and an insulator. This difference can be interpreted in terms of band theory with the band gap in boron nitride being considerably greater than that in graphite because of the polarity of the B_N bond.
Heating the layered form of BN at ≈ 2000K and >50 kbar pressure in the presence of catalytic amounts of Li3N or Mg3N2 converts it to a more dense polymorph, cubic-BN, with the zinc blende structure.
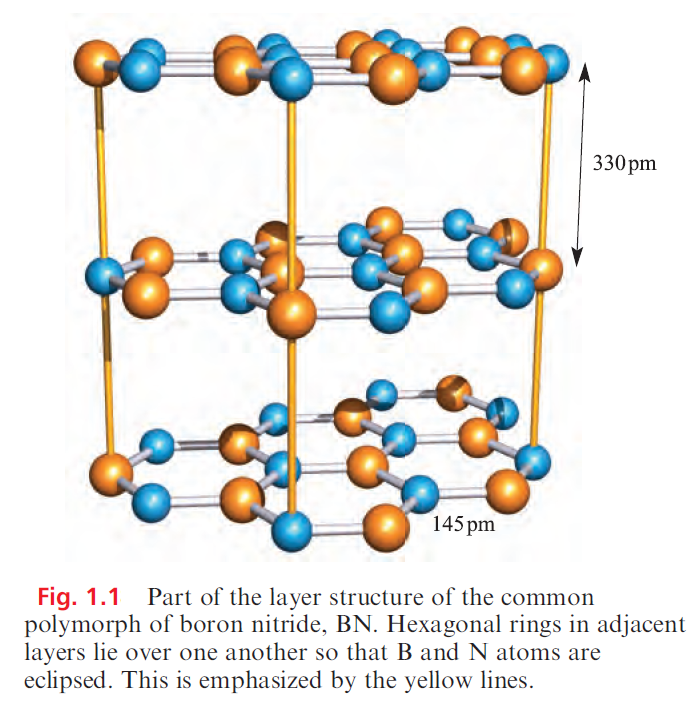
Table 1.1 shows that the B_N bond distance in cubic-BN is similar to those in R3N.BR3 adducts and longer than in the layered form of boron nitride; this further supports the existence of π-bonding within the layers of the latter. Structurally, the cubic form of BN resembles diamond and the two materials are almost equally hard; crystalline cubic BN is called borazon and is used as an abrasive. A third polymorph of boron nitride with a wurtzite lattice is formed by compression of the layered form at ≈12 kbar.
Of the group 13 metals, only Al reacts directly with N2 (at 1020 K) to form a nitride; AlN has a wurtzite lattice and is hydrolysed to NH3 by hot dilute alkali. Gallium and indium nitrides also crystallize with the wurtzite structure, and are more reactive than their B or Al counterparts. The importance of the group 13 metal nitrides, and of the related MP, MAs and MSb (M = Al, Ga, In) compounds, lies in their applications in the semiconductor industry (see also Section 18.4).
 الاكثر قراءة في الجدول الدوري وخواص العناصر
الاكثر قراءة في الجدول الدوري وخواص العناصر
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















