

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Neutral hydrides of group 13 elements
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المصدر:
Inorganic Chemistry
الجزء والصفحة:
p 301
28-1-2018
1946
Neutral hydrides of group 13 elements
With three valence electrons, each group 13 element might be expected to form a hydride MH3. Although the existence of BH3 has been established in the gas phase, its propensity to dimerize means that B2H6 (diborane(6)) is, in practice, the simplest hydride of boron.
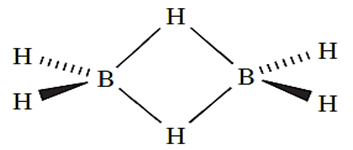
We have already discussed the structure of and bonding in B2H6 the reader is reminded of the presence of 3c-2e (delocalized, 3-centre 2-electron) B_H_B interactions.
Diborane(6) is an important reagent in synthetic organic chemistry, and reaction showen in below is one convenient laboratory preparation..
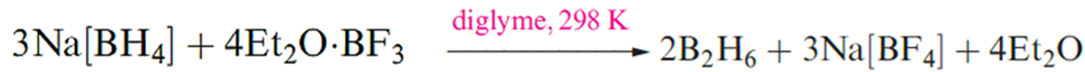
Although this reaction is standard procedure for the preparation of B2H6, it is not without problems. For example, the reaction temperature must be carefully controlled because the solubility of Na[BH4] in diglyme varies significantly with temperature. Secondly, the solvent cannot easily be recycled. Reaction that showen in below , which uses a triglyme adduct of BF3 as precursor, produces B2H6 quantitatively and is an improvement on the traditional reaction showen in above . Reaction below can be applied to large-scale syntheses, and the triglyme solvent can be recycled. Tetraglyme can be used in place of triglyme in below reaction .
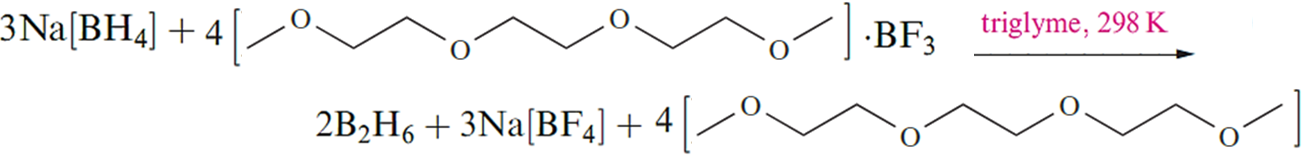
Reaction 12.10 is the basis for an industrial synthesis of B2H6.

Diborane(6) is a colourless gas (bp 180.5 K) which is rapidly decomposed by wate). Like other boron hydrides B2H6 has a small positive value of Δf Ho (36 kJ mol_1); mixtures with air or O2 are liable to inflame or explode.
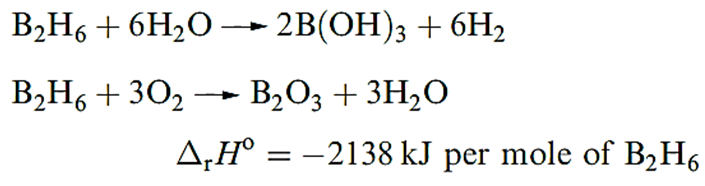
 الاكثر قراءة في الجدول الدوري وخواص العناصر
الاكثر قراءة في الجدول الدوري وخواص العناصر
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















