
Oxides, peroxides, superoxides, suboxides and ozonides of group 1
 المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
 المصدر:
Inorganic Chemistry
المصدر:
Inorganic Chemistry
 الجزء والصفحة:
p 264
الجزء والصفحة:
p 264
 15-1-2018
15-1-2018
 2788
2788
Oxides, peroxides, superoxides, suboxides and ozonides of group 1
When the group 1 metals are heated in an excess of air or in O2, the principal products obtained depend on the metal: lithium oxide, Li2O sodium peroxide, Na2O2 and the superoxides KO2, RbO2 and CsO2.
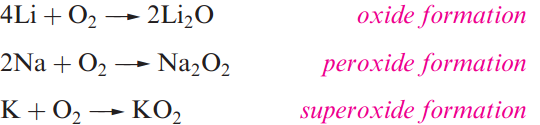
The oxides Na2O, K2O, Rb2O and Cs2O can be obtained impure by using a limited air supply, but are better prepared by thermal decomposition of the peroxides or superoxides.
The colours of the oxides vary from white to orange; Li2O and Na2O form white crystals while K2O is pale yellow, Rb2O yellow and Cs2O orange. All the oxides are strong bases, the basicity increasing from Li2O to Cs2O. A peroxide of lithium can be obtained by the action of H2O2 on an ethanolic solution of LiOH, but it decomposes on heating.
Sodium peroxide (widely used as an oxidizing agent) is manufactured by heating Na metal on Al trays in air; when pure, Na2O2 is colourless and the faint yellow colour usually observed is due to the presence of small amounts of NaO2. The superoxides and peroxides contain the paramagnetic [O2]- and diamagnetic [O2]2- ions respectively. Superoxides have magnetic moments of ≈1.73µB consistent with one unpaired electron.
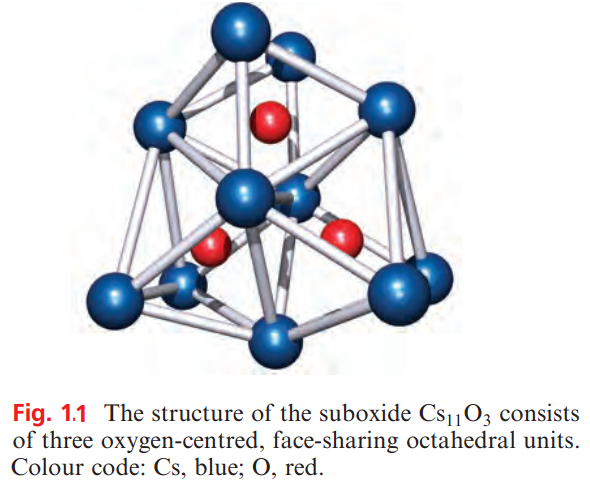
Partial oxidation of Rb and Cs at low temperatures yields suboxides such as Rb6O, Rb9O2, Cs7 O and Cs11O3. Their structures consist of octahedral units of metal ions with the oxygen residing at the centre; the octahedra are fused together by sharing faces. The formulae of the suboxides are misleading in terms of the oxidation states. Each contains M+ and O2_ ions, and, for example, the formula of Rb6O is better written as (Rb+)6(O2-).4e-, indicating the presence of free electrons.
The alkali metal oxides, peroxides and superoxides react with water. One use of KO2 is in breathing masks where it absorbs H2O producing O2 for respiration and KOH, which absorbs exhaled CO2 .
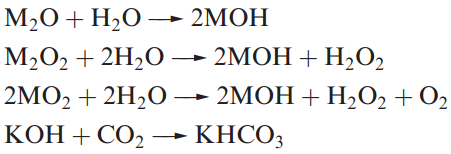
Sodium peroxide reacts with CO2 to give Na2CO3, rendering it suitable for use in air purification in confined spaces (e.g. in submarines); KO2 acts similarly but more effectively.
Although all the group 1 peroxides decompose on heating their thermal stabilities depend on cation size; Li2O2 is the least stable peroxide, while Cs2O2 is the most stable. The stabilities of the superoxides (with respect to decomposition to M2O2 and O2) follow a similar trend.
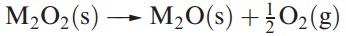
Ozonides,MO3, containing the paramagnetic, bent [O3]- ion are known for all the alkali metals. The salts KO3, RbO3 and CsO3 can be prepared from the peroxides or superoxides by reaction with ozone, but this method fails, or gives low yields, for LiO3 and NaO3. These ozonides have recently been prepared in liquid ammonia by the interaction of CsO3 with an ion-exchange resin loaded with either Li+ or Na+ ions. The ozonides are violently explosive.
 الاكثر قراءة في الجدول الدوري وخواص العناصر
الاكثر قراءة في الجدول الدوري وخواص العناصر
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


