

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
The hydrogen ion (proton)
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المصدر:
Organic Chemistry
الجزء والصفحة:
p 236
1-1-2018
1631
The hydrogen ion (proton)
The ionization energy of hydrogen (defined for reaction 1.1) is 1312 kJ mol‑1, a value that is high enough to preclude the existence of H+ ions under ordinary conditions.
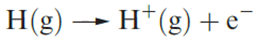 (1.1)
(1.1)
However, as we discussed in Chapter 6, the hydrated proton or oxonium ion, [H3O]+, is an important species in aqueous solution; ΔhydHo)H;g( = _1091 kJ mol_1 (see Section 6.9). The [H3O]+ ion (1.1) is a well-defined species which has been crystallographically characterized in various salts. The ions [H5O2] (Figure 1.1) and ½H9O4_ have also been isolated in crystalline acid hydrates. The [H5O2] and [H9O4] ions are members of the general family of hydrated protons [H(H2O)n] (n =1 to ≈ 20) and we return to these ions when we discuss hydrogen bonding in Section 9.6.
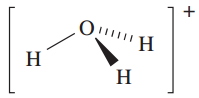
(1.1)
When crystals of a compound are grown from a solvent, they may contain solvent of crystallization; if the solvent is water, the compound is a hydrate. The formula of the solvated compound shows the molar ratio in which the solvent of crystallization is present, e.g. CuSO4.5H2O, copper(II) sulfate pentahydrate or copper(II) sulfate–water (1/5).
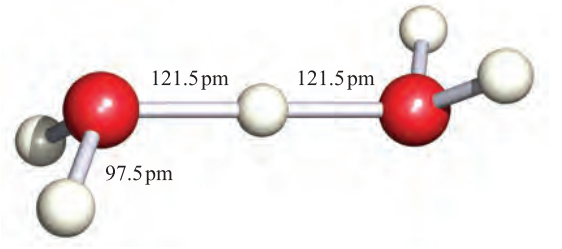
Fig. 1.1 The structure of [H5O2]+ determined by neutron diffraction in the compound [V(H2O) 6][H5O2][CF3SO3]4. [F.A. Cotton et al. (1984) J. Am. Chem. Soc., vol. 106, p. 5319.]
 الاكثر قراءة في الجدول الدوري وخواص العناصر
الاكثر قراءة في الجدول الدوري وخواص العناصر
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















