

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Osmotic Pressure
المؤلف:
Jerome L. Rosenberg and Lawrence M. Epstein
المصدر:
College Chemistry
الجزء والصفحة:
p 96
16-7-2017
2192
Osmotic Pressure
If a solution is separated from pure solvent by a semipermeable membrane that allows solvent, but not solute, molecules to pass through, solvent will move into the solution in an attempt to equalize the concentrations on the two sides of the membrane. This solvent transport process is called osmotic flow.
If there is no resisting force, solvent will continue to flow into the solution until the solvent reservoir is exhausted. However, if the solutions are arranged as shown in Figure 1.1, the incoming solvent will force the solution up the extension tube. The weight of this solution in the tube exerts a downward pressure that tends to oppose the flow of solvent. Eventually this pressure will exactly balance the force of solvent flow and equilibrium is attained. This equilibrium pressure is called the osmotic pressure.
The osmotic pressure P of a dilute solution of a nonelectrolyte is given by an equation formally equivalent to the ideal gas law:
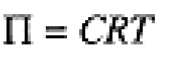 (1.1)
(1.1)
If C is the molar concentration of solute, T in K and R = 0.0821 L.atm/mol.K, P will have units of atm. Osmotic pressure measurements are particularly convenient for determination of the molar mass of macromolecules such as proteins.

Figure 1.1. Osmotic Pressure Apparatus
Example
A solution was prepared by dissolving 0.750 g of crab hemocyanin in 125 mL of water. At 4°C, the osmotic pressure corresponded to a rise of 2.6 mm of the solution (d = 1.00 g/mL). Determine the molar mass of the protein. We first convert the measured quantities to SI units and compute the osmotic pressure in pascals:
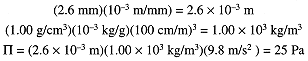
Then convert P to atm and compute the concentration using eq (1.1)
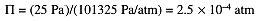
Finally compute the molar mass:
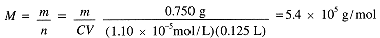
 الاكثر قراءة في مواضيع عامة في الكيمياء الفيزيائية
الاكثر قراءة في مواضيع عامة في الكيمياء الفيزيائية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















