

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Atomic Radii
المؤلف:
Jerome L. Rosenberg and Lawrence M. Epstein
المصدر:
College Chemistry
الجزء والصفحة:
p 56
4-7-2017
2190
Atomic Radii
The electron cloud around an atomic nucleus makes the concept of atomic size somewhat imprecise, but it is useful to refer to an atomic radius. One can arbitrarily divide the distance between centers of two bonded atoms to arrive at two radii, based on the crude picture that two bonded atoms are spheres in contact. If the bonding is covalent, the radius is called a covalent radius (see Table 8-2); if it is ionic, the radius is an ionic radius . The radius for non-bonded atoms may be defined in terms of the distance of closest non-bonding approach; such a measure is called the van der Waals radius. These three concepts of size are illustrated in Figure 1.1.
Generalizations regarding atomic size: (1) Within a column of the periodic table, radii increase with increasing atomic number, a result of the increasing value of n for the valence electrons. (2) Within a given period (row) of the table, covalent radii generally decrease with increasing Z; n is constant across the period, but the nuclear charge increases. (3) A cation radius is small compared with the covalent radius of the neutral atom since one or more valence electrons has been removed. (4) An anion radius is significantly greater than the covalent radius of the neutral atom since the extra electron(s) are held less tightly (more electrons, but the same nuclear charge).
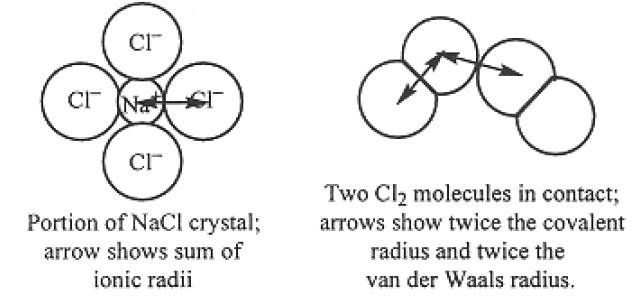
Figure 1.1. Illustrations of ionic, covalent, and van der Waals radii.
 الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















