

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Balancing Chemical Equations
المؤلف:
Jerome L. Rosenberg and Lawrence M. Epstein
المصدر:
College Chemistry
الجزء والصفحة:
p 20
24-6-2017
2404
Balancing Chemical Equations
A balanced equation is the basis for all calculations of the quantities of substances involved in a chemical reaction. In the course of balancing equations and working the related numerical problems, the alert student will not only develop skill in calculation, but will acquire much knowledge of descriptive and practical chemistry.
When substances—the reactants—react to form new substances—the products—we say that a chemical reaction has occurred. A chemical equation is a statement of such an event in which the formulas of the reactants are on the left, followed by a right-pointing arrow and then the formulas of the products. In a balanced equation, coefficients specify the number of molecules (or formula units) of each species involved. The coefficients must satisfy Dalton's requirement that atoms are not created or destroyed in a chemical reaction. There is no fixed procedure for balancing an equation. Although a systematic algebraic approach is in principle possible, a trial-and-error approach often works.
Example 1
Balance: FeS2(s) + O2(g) → Fe2O3(s) + SO2(g)
We start with an algebraic approach, writing the unknown integral coefficients as w, x, y and z:

Dalton's rule leads to conservation relations for Fe, S and O:

Since 2x and 2z are necessarily even numbers, we see that y must also be even; let us guess y = 2. The three equations then give w = 4, z = 8, and x = 11. The complete equation then is
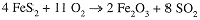
The trial-and-error approach also works well. We can start by focusing on one element, say Fe. Since Fe2O3 has two Fe atoms, we can set the coefficient of FeS2 to 2, obtaining

Next we balance S; since 2 FeS2 contains 4 S atoms, the coefficient of SO2 must be 4
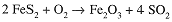
Finally we balance O. The right-hand side has 3 + 8 = 11 O atoms. The equation could be balanced by using a coefficient 11/2 for O2, but since we want integral coefficients, we multiply through by 2 to obtain the same result obtained by the algebraic approach.
Example 2
Balance: C7H6O2(s) + O2(g) → CO2(g) + H2O(l)
This time, we use the trial-and-error approach. With 7 C on the left, we must have 7 on the right:

With 6 H on the left, we must have 6 on the right:
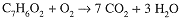
We now have 7 × 2 + 3 = 17 O atoms on the right which, in view of 2 O atoms in C7H6O2, suggests a coefficient 15/2 for O2 on the left. Again multiplying through by 2, we have

 الاكثر قراءة في مواضيع عامة في الكيمياء التحليلية
الاكثر قراءة في مواضيع عامة في الكيمياء التحليلية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















