
Radioactive Half-Life
 المؤلف:
U.S. Department of Commerce, National Technical Information Service, 1993
المؤلف:
U.S. Department of Commerce, National Technical Information Service, 1993
 المصدر:
The Nuclear Physics and Reactor Theory Handbook
المصدر:
The Nuclear Physics and Reactor Theory Handbook
 الجزء والصفحة:
p 32
الجزء والصفحة:
p 32
 28-3-2017
28-3-2017
 1999
1999
Radioactive Half-Life
One of the most useful terms for estimating how quickly a nuclide will decay is the radioactive half-life. The radioactive half-life is defined as the amount of time required for the activity to decrease to one-half of its original value. A relationship between the half-life and decay constant can be developed from Equation (1-1). The half-life can be calculated by solving Equation (1-1) for the time, t, when the current activity, A, equals one-half the initial activity Ao .
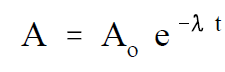 (1-1)
(1-1)
First, solve Equation (1-1) for t.

If A is equal to one-half of Ao , then A/Ao is equal to one-half. Substituting this in the equation above yields an expression for t1/2 .
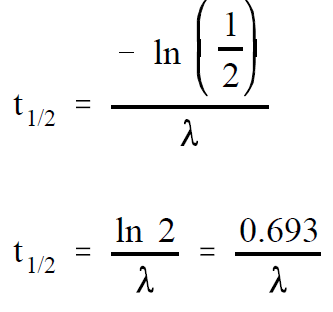 (1-2)
(1-2)
The basic features of decay of a radionuclide sample are shown by the graph in Figure 1.
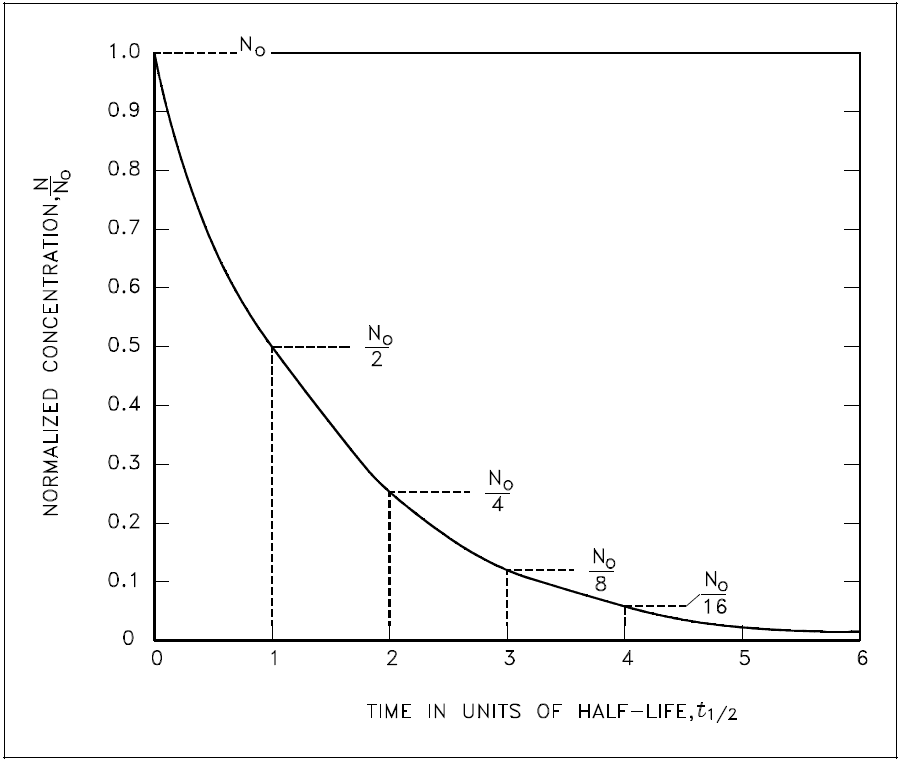
Figure 1: Radioactive Decay as a Function of Time in Units of Half-Life
Assuming an initial number of atoms No, the population, and consequently, the activity may be noted to decrease by one-half of this value in a time of one half-life. Additional decreases occur so that whenever one half-life elapses, the number of atoms drops to one-half of what its value was at the beginning of that time interval. After five half-lives have elapsed, only 1/32, or 3.1%, of the original number of atoms remains. After seven half-lives, only 1/128, or 0.78%, of the atoms remains. The number of atoms existing after 5 to 7 half-lives can usually be assumed to be negligible. The Chemistry Fundamentals Handbook contains additional information on calculating the number of atoms contained within a sample.
 الاكثر قراءة في النشاط الاشعاعي
الاكثر قراءة في النشاط الاشعاعي
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


