

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Fullerenes: reactivity
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المصدر:
INORGANIC CHEMISTRY
الجزء والصفحة:
2th ed p 349
27-3-2017
3408
Fullerenes: reactivity
Since efficient syntheses have been available, fullerenes (in particular C60) have been the focus of an explosion of research. We provide a brief introduction to the chemical properties of C60; organometallic derivatives are covered in Section 23.10, and the reading list at the end of the chapter gives more in-depth coverage.
The structural representation in Figure 1.1b suggests connected benzene rings, but the chemistry of C60 is not reminiscent of benzene. Although C60 exhibits a small degree of aromatic character, its reactions tend to reflect the presence of localized double and single C_C bonds, e.g. C60 undergoes addition reactions.
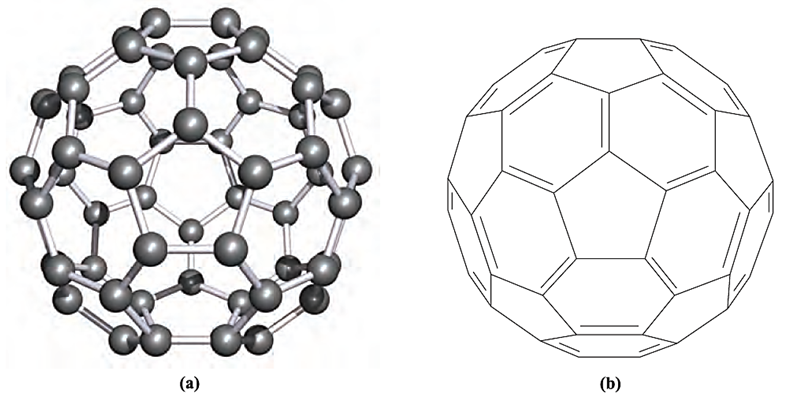
Fig. 1.1 (a) The structure of the fullerene C60; the approximately spherical molecule is composed of fused 5- and 6-membered rings of carbon atoms. [X-ray diffraction at 173K of the benzene solvate C60 4C6H6, M.F. Meidine et al. (1992) J. Chem. Soc., Chem. Commun., p. 1534.] (b) A representation of C60, in the same orientation as is shown in (a), but showing only the upper surface and illustrating the localized single and double carbon–carbon bonds.
Birch reduction gives a mixture of polyhydrofullerenes (equation 1.1) with C60H32 being the dominant product; reoxidation occurs with the quinone shown. Reaction 1.2 shows a selective route to C60H36; the hydrogen-transfer agent is 9,10-dihydroanthracene (DHA).
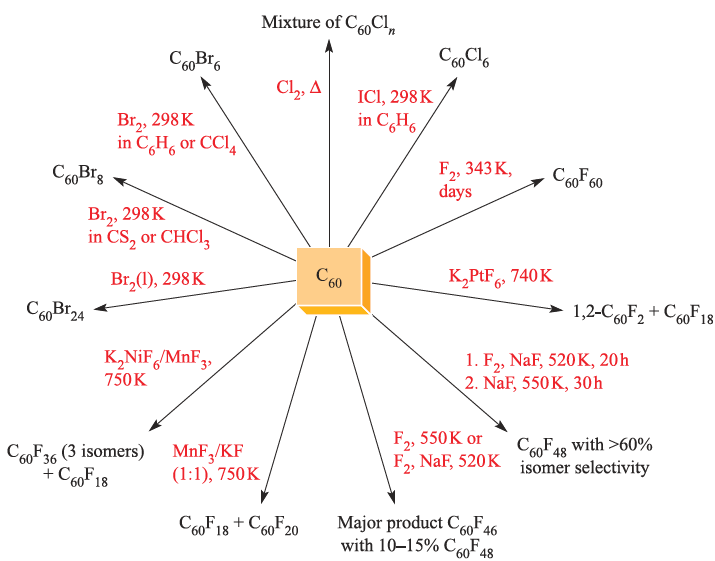
Fig. 1.2 Halogenation reactions of C60. Although the number of possible isomers for products C60Xn where 2 ≤ n ≤ 58 is, at the very least, large, some of the reactions (such as fluorination using NaF and F2) are surprisingly selective.
In addition to being a selective method of hydrogenation, use of 9,9’,10,10’-[D4]dihydroanthracene provides a method of selective deuteration.
 (1.1)
(1.1)
 (1.2)
(1.2)
Additions of F2, Cl2 and Br2 also occur, the degree and selectivity of halogenation depending on conditions (Figure 1.2). Because F atoms are small, addition of F2 to adjacent C atoms in C60 is possible, e.g. to form 1,2-C60F2. However, in the addition of Cl2 or Br2, the halogen atoms prefer to add to remote C atoms. Thus, in C60Br8 and in C60Br24 (Figure 1.3a), the Br atoms are in 1,3- or 1,4-positions with respect to each other. Just as going from benzene to cyclohexane causes a change from a planar to boat- or chair-shaped ring, addition of substituents to C60 causes deformation of the near-spherical surface. This is illustrated in Figure 1.3 with the structures of C60Br24 and C60F18. The C60-cage in C60Br24 includes both boat and chair C6-rings.
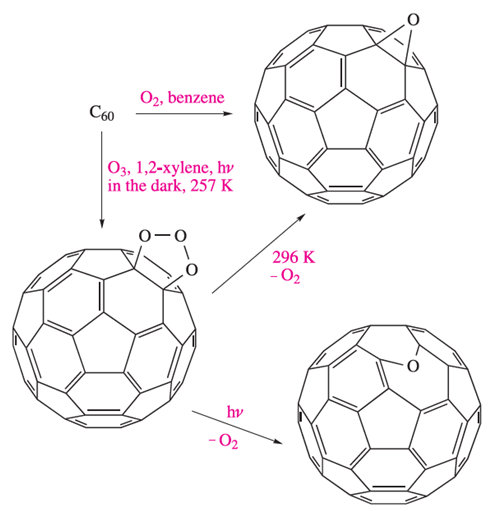
Addition of a Br to a C atom causes a change from sp2 to sp3 hybridization. The arrangement of the Br atoms over the surface of the C60 cage is such that they are relatively far apart from each other. In contrast, in C60F18 (Figure 1.3b), the F atoms are in 1,2-positions with respect to each other and the C60-cage suffers severe ‘flattening’ on the side associated with fluorine addition. At the centre of the flattened part of the cage lies a planar, C6-ring (shown at the centre of the lower part of Figure 1.3b). This ring has equal C–C bond lengths (137 pm) and has aromatic character. It is surrounded by sp3 hybridized C atoms, each of which bears an F atom. The ene-like nature of C60 is reflected in a range of reactions such as the additions of an O atom to give an epoxide (C60O) and of O3 at 257K to yield an intermediate ozonide (C60O3). In hydrocarbon solvents, addition occurs at the junction of two 6-membered rings (a 6,6-bond), i.e. at a C=C bond, as shown in scheme 1.1. Loss of O2 from C60O3 gives C60O but the structure of this product depends on the reaction conditions. At 296 K, the product is an epoxide with the O bonded across a 6,6-bond. In contrast, photolysis opens the cage and the O atom bridges a 5,6-edge (scheme 1.1).
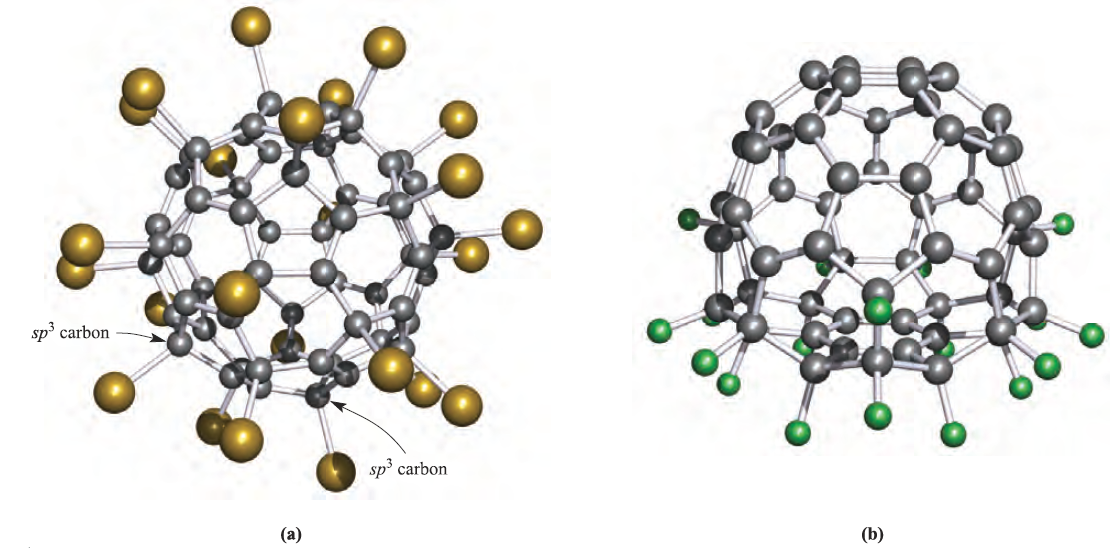
Fig. 1.3 The structure of C60Br24 determined by X-ray diffraction at 143K [F.N. Tebbe et al. (1992) Science, vol. 256, p. 822]. The introduction of substituents results in deformation of the C60 surface. (b) The structure (X-ray diffraction at 100 K) of C60F18 [I.S. Neretin et al. (2000) Angew. Chem. Int. Ed., vol. 39, p. 3273]. Note that the F atoms are all associated with the ‘flattened’ part of the fullerene cage. Colour code: C, grey; Br, gold; F, green.
 (1.1)
(1.1)
Other reactions typical of double-bond character include the formation of cycloaddition products (exemplified schematically in equation 1.2), and some have been developed to prepare a range of rather exotic derivatives.
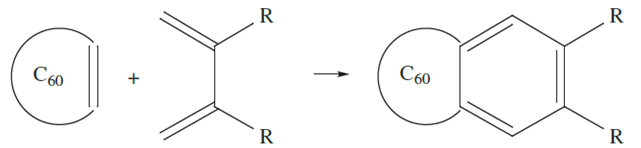 (1.2)
(1.2)
Reactions of C60 with free radicals readily occur, e.g. photolysis of RSSR produces RS* which reacts with C60 to give C60SR˙, although this is unstable with respect to regeneration of C60. The stabilities of radical species C60Y˙ are highly dependent on the steric demands of Y. When the reaction of t-Bu* (produced by photolysis of a tert-butyl halide) with C60 is monitored by ESR spectroscopy (which detects the presence of unpaired electrons), the intensity of the signal due to the radical C60 t-Bu* increases over the temperature range 300–400 K. These data are consistent with equilibrium 1.3, with reversible formation and cleavage of an inter-cage C_C bond.
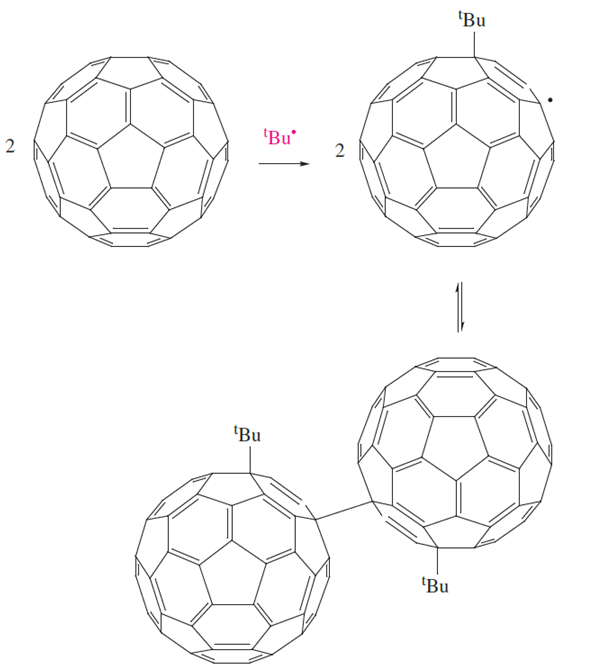 (1.3)
(1.3)
The formation of methanofullerenes, C60CR2, occurs by reaction at either 5,6- or 6,6-edges in C60. For the 6,6- addition products, the product of the reaction of C60 with diphenylazomethane is C61Ph2 (equation 1.5) and, initially, structural data suggested that the reaction was an example of ‘cage expansion’ with the addition of the CPh2 unit being concomitant with the cleavage of the C_C bond marked a in equation 1.5. This conclusion was at odds with NMR spectroscopic data and theoretical calculations, and a low-temperature X-ray diffraction study of compound 1.1 has confirmed that 6,6-edge-bridged methanofullerenes should be described in terms of the C60 cage sharing a common C_C bond with a cyclopropane ring.
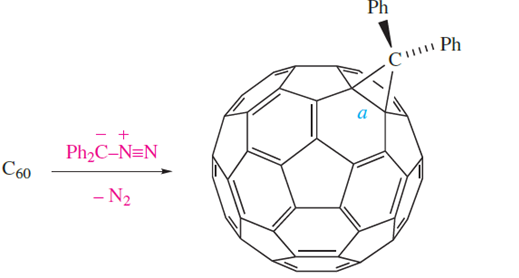 (1.5)
(1.5)

(1.1)
Theoretical studies on C60 show that the LUMO is triply degenerate and the HOMO–LUMO separation is relatively small. It follows that reduction of C60 should be readily achieved. A number of charge transfer complexes have been prepared in which a suitable donor molecule transfers an electron to C60 as in equation 1.6. This particular product is of importance because, on cooling to 16 K, it becomes ferromagnetic (see Figure 20.25).
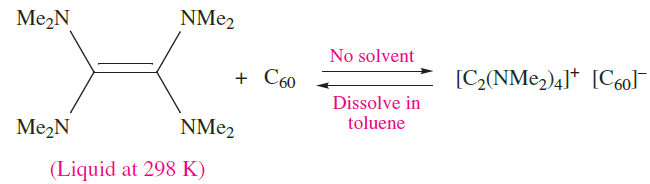 (1.6)
(1.6)
The electrochemical reduction of C60 results in the formation of a series of fulleride ions, [C60]n- where n = 1–6. The midpoint potentials (obtained using cyclic voltammetry and measured with respect to the ferrocenium/ferrocene couple, Fc+/Fc = 0 V, ferrocene; see Section 23.13) for the reversible one-electron steps at 213K are given in scheme 1.7.
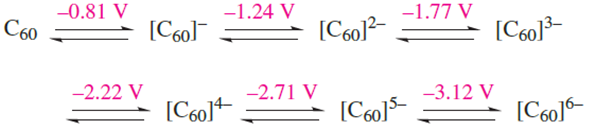 (1.7)
(1.7)
By titrating C60 in liquid NH3 against an Rb/NH3 solution (see Section 8.6) at 213 K, five successive reduction steps are observed and the [C60]n- anions have been studied by vibrational and electronic spectroscopies. At low temperatures, some alkali metal fulleride salts of type [M]3+[C60]3- become superconducting (see Section 27.4). The structures of the M3C60 fullerides can be described in terms of M+ ions occupying the interstitial holes in a lattice composed of close-packed, near-spherical C60 cages. In K3C60 and Rb3C60, the [C60]3- cages are arranged in a ccp lattice, and the cations fully occupy the octahedral and tetrahedral holes (Figure 1.4). The temperature at which a material becomes superconducting is its critical temperature, Tc. Values of Tc for K3C60 and Rb3C60 are 18K and 28K respectively, and for Cs3C60 (in which the C60 cages adopt a bcc lattice), Tc = 40 K. Although Na3C60 is structurally related to K3C60 and Rb3C60, it is not superconducting. The paramagnetic [C60]2- anion has been isolated as the [K(crypt-222)] salt (reaction 1.8 and Section 10.8).
 (1.8)
(1.8)

Fig. 1.4 A representation of the structures of K3C60 and Rb3C60 in which the [C60] 3- cages are arranged in an fcc lattice with the M ions occupying all the octahedral (grey) and tetrahedral (red) holes. Some of the cations in the unit cell shown are hidden by [C60]3- anions.
In the solid state, the [C60]2- cages are arranged in layers with hexagonal packing, although the cages are well separated; [K(crypt-222)] cations reside between the layers of fulleride anions. The coupling of C60 molecules through [2 + 2] cycloaddition to giveC120 (1.2) can be achieved by a solid state reaction that involves high-speed vibration milling of C60 in the presence of catalytic amounts of KCN. When heated at 450K for a short period, the C120 molecule dissociates into C60.
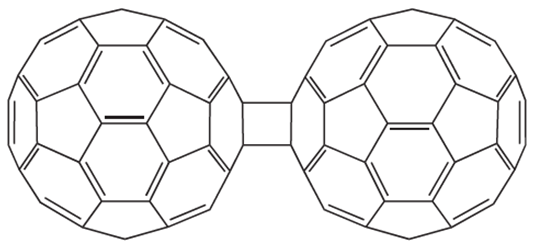
(1.2)
Endohedral metallofullerenes are a remarkable series of compounds in which metal atoms are encapsulated within a fullerene cage; the general family is denoted as Mx@Cn. Examples of these compounds include Sc2@C84, Y@C82, La2@C80 and Er@C60. In general, the larger fullerenes produce more stable compounds than C60. The compounds are prepared by vaporizing graphite rods impregnated with an appropriate metal oxide or metal carbide. By use of 13C and 139La NMR spectroscopies, it has been shown that the two lanthanum atoms in La2@C80 undergo circular motion within the fullerene cage.
 الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















