
Chart of the Nuclides
 المؤلف:
U.S. Department of Commerce, National Technical Information Service, 1993
المؤلف:
U.S. Department of Commerce, National Technical Information Service, 1993
 المصدر:
The Nuclear Physics and Reactor Theory Handbook
المصدر:
The Nuclear Physics and Reactor Theory Handbook
 الجزء والصفحة:
p 11
الجزء والصفحة:
p 11
 25-3-2017
25-3-2017
 2601
2601
Chart of the Nuclides
A tabulated chart called the Chart of the Nuclides lists the stable and unstable nuclides in addition to pertinent information about each one. Figure 1 shows a small portion of a typical chart. This chart plots a box for each individual nuclide, with the number of protons (Z) on the vertical axis and the number of neutrons (N = A - Z) on the horizontal axis.
The completely gray squares indicate stable isotopes. Those in white squares are artificially radioactive, meaning that they are produced by artificial techniques and do not occur naturally. By consulting a complete chart, other types of isotopes can be found, such as naturally occurring radioactive types (but none are found in the region of the chart that is illustrated in Figure 1).
Located in the box on the far left of each horizontal row is general information about the element. The box contains the chemical symbol of the element in addition to the average atomic weight of the naturally occurring substance and the average thermal neutron absorption cross section, which will be discussed in a later module. The known isotopes (elements with the same atomic number Z but different mass number A) of each element are listed to the right.
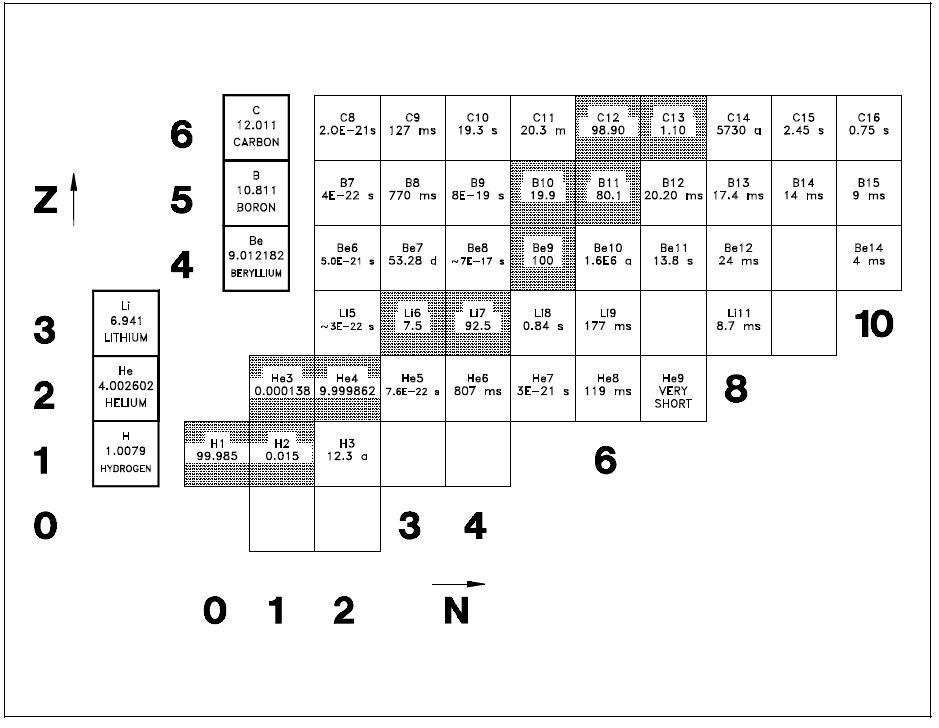
Figure 1: Nuclide Chart for Atomic Numbers 1 to 6
 الاكثر قراءة في مواضيع عامة في الفيزياء النووية
الاكثر قراءة في مواضيع عامة في الفيزياء النووية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


