

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Selenium and tellurium
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المصدر:
INORGANIC CHEMISTRY
الجزء والصفحة:
2th ed p 441
12-3-2017
1638
Selenium and tellurium
Selenium possesses several allotropes. Crystalline, red monoclinic selenium exists in three forms, each containing Se8 rings with the crown conformation of S8. Black selenium consists of larger polymeric rings, and the thermodynamically stable allotrope is grey selenium. Elemental selenium can be prepared by reaction 1.1. By substituting Ph3PSe in this reaction by Ph3PS, rings of composition SenS 8-n (n = 1–5) can be produced.
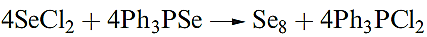 (1.1)
(1.1)
Tellurium has only one crystalline form which is a silverywhite metallic-looking solid. In both grey Se and Te, the atoms form infinite helical chains, the axes of which lie parallel to each other. The red allotropes of Se can be obtained by rapid cooling of molten Se and extraction into CS2. The photoconductivity of Se and Te arises because, in the solid, the band gap of 160 kJ mol-1 is small enough for the influence of visible light to cause the promotion of electrons from the filled bonding MOs to the unoccupied antibonding MOs. Although cyclo-Te8 is not known as an allotrope of the element, it has been characterized in the salt Cs3[Te22] which has the composition [Cs]3+[Te63-][Te8]2. Although less reactive, Se and Te are chemically similar to sulfur. This resemblance extends to the formation of cations such as [Se4]2+, [Te4]2+, [Se8]2+ and [Te8]2+. The salt [Se8][AsF6]2 can be made in an analogous manner to [S8][AsF6]2 in liquid SO2 (equation 15.21), whereas reaction 1.2 is carried out in fluorosulfonic acid. Recent methods use metal halides (e.g. ReCl4 and WCl6) as oxidizing agents, e.g. the formation of [Te8]2+ (equation 1.3). Reaction 1.4 (in AsF3 solvent) produces [Te6]4+, 1.1, which has no S or Se analogue.
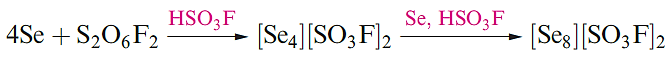 (1.2)
(1.2)
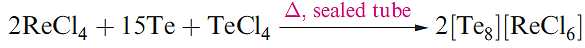 (1.3)
(1.3)
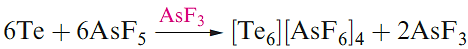 (1.4)
(1.4)
The structures of [Se4]2+, [Te4]2+ and [Se8]2+ mimic those of their S analogues, but [Te8]2+ exists in two forms. In [Te8][ReCl6], [Te8]2+ is structurally similar to [S8]2+ and [Se8]2+, but in [Te8][WCl6]2, the cation has the bicyclic structure, i.e. resonance structure 1.2 is dominant.
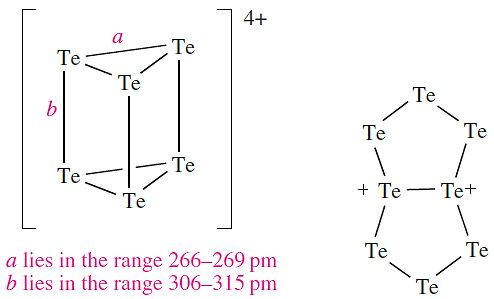
(1.1) (1.2)
 الاكثر قراءة في الجدول الدوري وخواص العناصر
الاكثر قراءة في الجدول الدوري وخواص العناصر
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















