

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Ozone
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المصدر:
INORGANIC CHEMISTRY
الجزء والصفحة:
2th ed p 438
12-3-2017
1500
Ozone
Ozone, O3, is usually prepared in up to 10% concentration by the action of a silent electrical discharge between two concentric metallized tubes in an apparatus called an ozonizer. Electrical discharges in thunderstorms convert O2 into ozone. The action of UV radiation on O2, or heating O2 above 2750K followed by rapid quenching, also produces O3. In each of these processes, O atoms are produced and combine with O2 molecules. Pure ozone can be separated from reaction mixtures by fractional liquefaction; the liquid is blue and boils at 163K to give a perceptibly blue gas with a characteristic ‘electric’ smell. Molecules of O3 are bent (Figure 1.1). Ozone absorbs strongly in the UV region, and its presence in the upper atmosphere of the Earth is essential in protecting the planet’s surface from over-exposure to UV radiation from the Sun. Ozone is highly endothermic (equation 1.1). The pure liquid is dangerously explosive, and the gas is a very powerful oxidizing agent (equation 1.2).
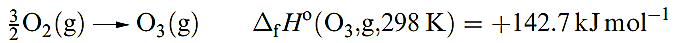 (1.1)
(1.1)
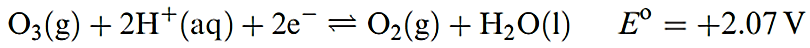 (1.2)
(1.2)
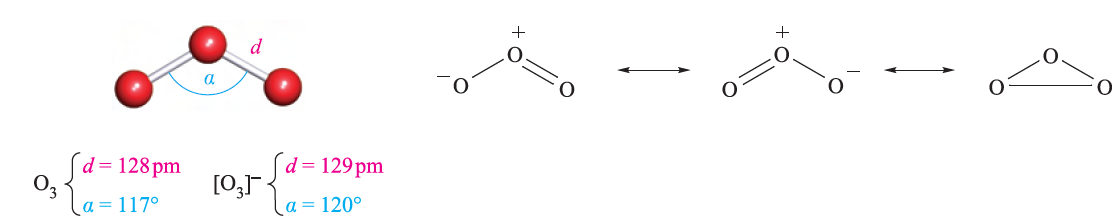
Fig. 1.1 The structures of O3 and [O3]-, and contributing resonance structures in O3. The O_O bond order in O3 is taken to be 1.5.
The value of Eo in equation 1.2 refers to pH = 0, and at higher pH, E diminishes: 1.65V at pH = 7, and 1.24V at pH = 14. The presence of high concentrations of alkali stabilizes O3 both thermodynamically and kinetically. Ozone is much more reactive than O2 (hence the use of O3 in water purification). Reactions 1.3–1.5 typify this high reactivity, as does its reaction with alkenes to give ozonides.
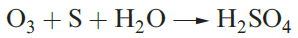 (1.3)
(1.3)
 (1.4)
(1.4)
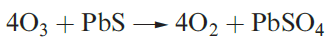 (1.5)
(1.5)
Potassium ozonide, KO3 (formed in reaction 1.6), is an unstable red salt which contains the paramagnetic [O3]- ion (Figure 1.1). Ozonide salts are known for all the alkali metals. The compounds [Me4N][O3] and [Et4N][O3] have been prepared using reactions of the type shown in equation 1.7. Ozonides are explosive, but [Me4N][O3] is relatively stable, decomposing above 348K.
 (1.6)
(1.6)
 (1.7)
(1.7)
Phosphite ozonides, (RO)3PO3, have been known since the early 1960s, and are made in situ as precursors to singlet oxygen. The ozonides are stable only at low temperatures, and it is only with the use of modern low-temperature crystallographic methods that structural data are now available. Figure 1.1 shows the structure of the phosphite ozonide
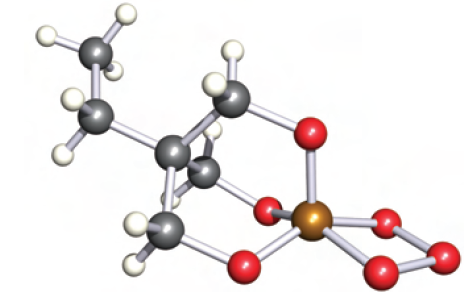
Fig. 1.1 The structure (X-ray diffraction at 188 K) of the phosphite ozonide EtC(CH2O)3PO3 [A. Dimitrov et al. (2001) Eur. J. Inorg. Chem., p. 1929]. Colour code: P, brown; O, red; C, grey; H, white.
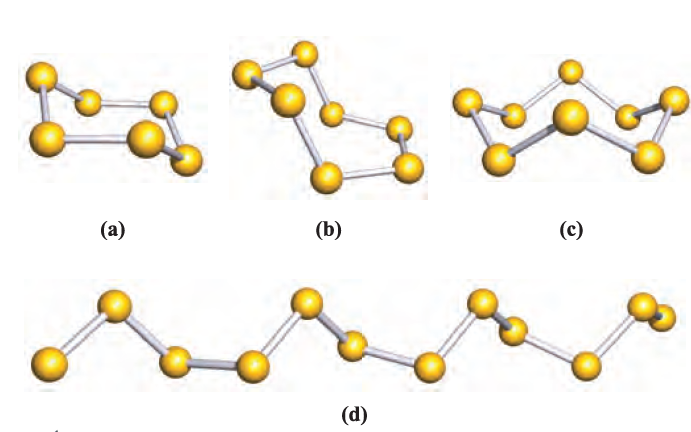
Fig. 1.2 Schematic representations of the structures of some allotropes of sulfur: (a) S6, (b) S7, (c) S8 and (d) catena-S1 (the chain continues at each end).
prepared by the steps in scheme 1.8. In the PO3 ring, the P_O and O_O bond lengths are 167 and 146 pm, respectively; the ring is close to planar, with a dihedral angle of 78.
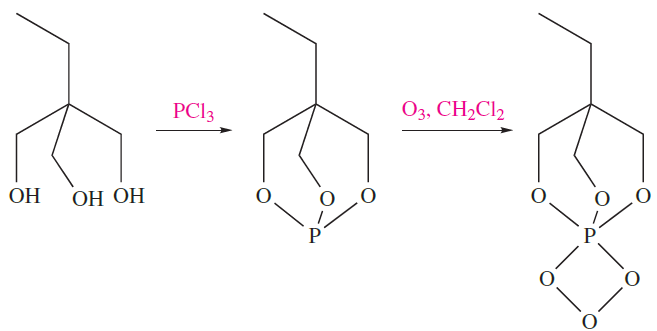
(1.8)
 الاكثر قراءة في الجدول الدوري وخواص العناصر
الاكثر قراءة في الجدول الدوري وخواص العناصر
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















