

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
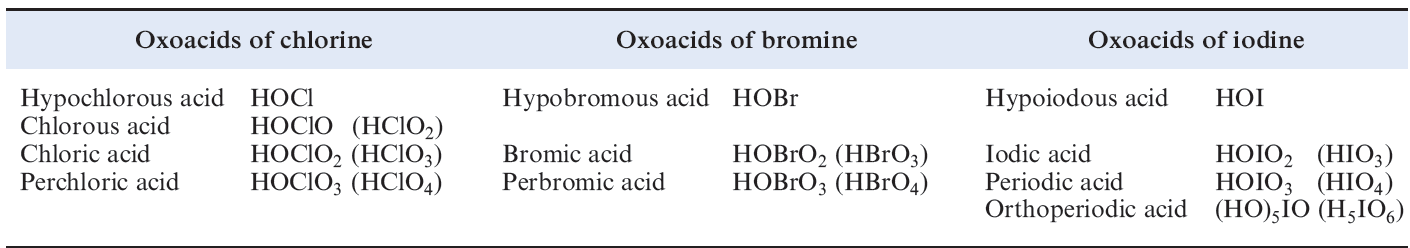
Oxoacids of chlorine, bromine and iodine
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المصدر:
INORGANIC CHEMISTRY
الجزء والصفحة:
2th ed p 485
11-3-2017
6136
Oxoacids of chlorine, bromine and iodine
Table 1.1 lists the families of oxoacids known for Cl, Br and I.
Table 1.1 Oxoacids of chlorine, bromine and iodine.
The hypohalous acids, HOX, are obtained in aqueous solution by reaction 1.1 (compare reactions 1.9 and 16.42).
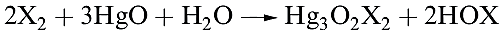 (1.1)
(1.1)
All are unknown as isolated compounds, but act as weak acids in aqueous solutions (pKa values: HOCl, 4.53; HOBr, 8.69; HOI, 10.64). Hypochlorite salts such as NaOCl, KOCl and Ca(OCl)2 (equation 1.2) can be isolated; NaOCl can be crystallized from a solution obtained by electrolysing aqueous NaCl in such a way that the Cl2 liberated at the anode mixes with the NaOH produced at the cathode. Hypochlorites are powerful oxidizing agents and in the presence of alkali convert [IO3]- to [IO4]-, Cr3+ to [CrO4]2- , and even Fe3+ to [FeO4]2-. Bleaching powder is a non-deliquescent mixture of CaCl2, Ca(OH)2 and Ca(OCl)2 and is manufactured by the action of Cl2 on Ca(OH)2; NaOCl is a bleaching agent and disinfectant.
 (1.2)
(1.2)
All hypohalites are unstable with respect to disproportionation (equation 1.3); at 298 K, the reaction is slow for [OCl]-, fast for [OBr]- and very fast for [OI]- . Sodium hypochlorite disproportionates in hot aqueous solution (equation 1.4), and the passage of Cl2 through hot aqueous alkali yields chlorate and chloride salts rather than hypochlorites. Hypochlorite solutions decompose by reaction 1.5 in the presence of cobalt(II) compounds as catalysts.
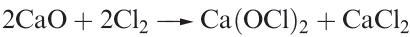 (1.3)
(1.3)
 (1.4)
(1.4)
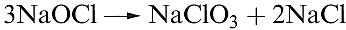 (1.5)
(1.5)
Like HOCl, chlorous acid, HClO2, is not isolable but is known in aqueous solution and is prepared by reaction 1.6; it is weak acid (pKa = 2.0). Sodium chlorite (used as a bleach) is made by reaction 1.7; the chlorite ion has the bent structure 1.1.
 (1.6)
(1.6)
 (1.7)
(1.7)
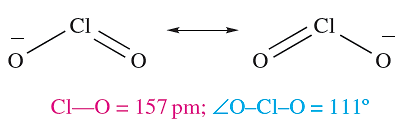
(1.1)
Alkaline solutions of chlorites persist unchanged over long periods, but in the presence of acid, a complex decomposition occurs which is summarized in equation 1.8.
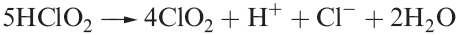 (1.8)
(1.8)
Chloric and bromic acids, HClO3 and HBrO3, are both strong acids but cannot be isolated as pure compounds. The aqueous acids can be made by reaction 1.9 (compare with reaction 1.6).
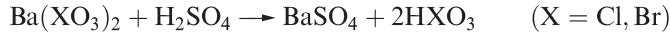 (1.9)
(1.9)
Iodic acid, HIO3, is a stable, white solid at room temperature, and is produced by reacting I2O5 with water (equation in below) or by the oxidation of I2 with nitric acid.
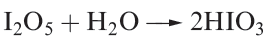
Crystalline iodic acid contains trigonal HIO3 molecules connected by extensive hydrogen bonding. In aqueous solution it is af airly strong acid (pKa = 0.77).
Chlorates are strong oxidizing agents; commercially, NaClO3 is used for the manufacture of ClO2 and is used as a weedkiller, and KClO3 has applications in fireworks and safety matches. Chlorates are produced by electrolysis of brine at 340 K, allowing the products to mix efficiently (scheme 1.10); chlorate salts are crystallized fromthe mixture.
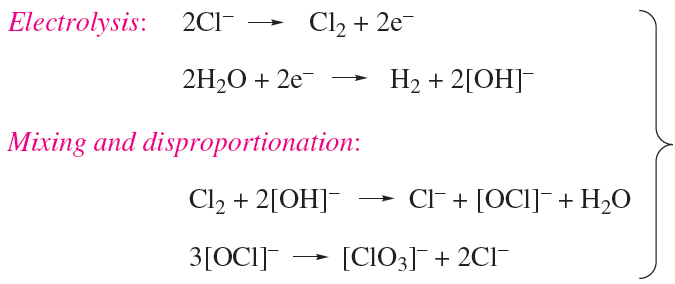 (1.10)
(1.10)
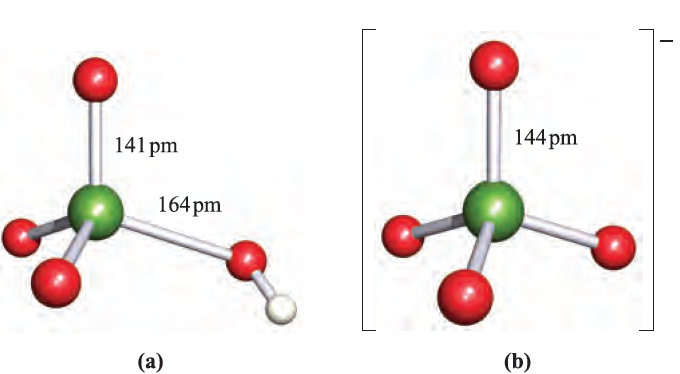
Fig. 1.1 Structures of (a) perchloric acid, in which one Cl_O bond is unique, and (b) perchlorate ion, in which all Cl_O bonds are equivalent. Colour code: Cl, green; O, red; H, white.
Anodic oxidation of [OCl]- produces further [ClO3]-. Bromates are made by, for example, reaction 1.11 under alkaline conditions. Reaction 1.12 is a convenient synthesis of KIO3.
 (1.11)
(1.11)
 (1.12)
(1.12)
Potassium bromate and iodate are commonly used in volumetric analysis. Very pure KIO3 is easily obtained, and reaction 1.13 is used as a source of I2 for the standardization of thiosulfate solutions (reaction 15.113).
 (1.13)
(1.13)
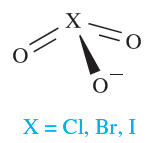
(1.2)
Halate ions are trigonal pyramidal (1.2) although, in the solid state, some metal iodates contain infinite structures in which two O atoms of each iodate ion bridge two metal centres.† The thermal decomposition of alkali metal chlorates follows reaction 1.14, but in the presence of a suitable catalyst, KClO3 decomposes to give O2. Some iodates (e.g. KIO3) decompose when heated to iodide and O2, but others (e.g. Ca(IO3)2) give oxide, I2 and O2. Bromates behave similarly and the interpretation of these observations is a difficult problem in energetics and kinetics.
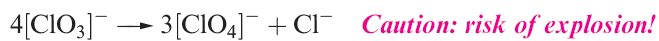 (1.14)
(1.14)
Perchloric acid is the only oxoacid of Cl that can be isolated, and its structure is shown in Figure 1.1a. It is a colourless liquid (bp 363K with some decomposition), made by heating KClO4 with concentrated H2SO4 under reduced pressure. Pure perchloric acid is liable to explode when heated or in the presence of organic material, but in dilute solution, [ClO4]- is very difficult to reduce despite the reduction potentials (which provide thermodynamic but not kinetic data) shown in equations 1.15 and 1.17. Zinc, for example, merely liberates H2, and iodide ion has no action.
Reduction to Cl- can be achieved by Ti(III) in acidic solution or by Fe(II) in the presence of alkali.
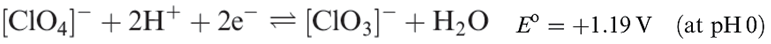 (1.15)
(1.15)
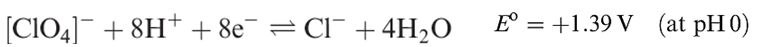 (1.17)
(1.17)
Perchloric acid is an extremely strong acid in aqueous solution . Although [ClO4]- (Figure 1.1b) does form complexes with metal cations, the tendency to do so is less than for other common anions. Consequently, NaClO4 solution is a standard medium for the investigation of ionic equilibria in aqueous systems. Alkali metal perchlorates can be obtained by disproportionation of chlorates (equation 1.14) under carefully controlled conditions; traces of impurities can catalyse decomposition to chloride and O2. Perchlorate salts are potentially explosive and must be handled with particular care; mixtures of ammonium perchlorate and aluminium are standard missile propellants, e.g. in the space shuttle. When heated, KClO4 gives KCl and O2, apparently without intermediate formation of KClO3. Silver perchlorate, like silver salts of some other very strong acids (e.g. AgBF4, AgSbF6 and AgO2CCF3), is soluble in many organic solvents including C6H6 and Et2O owing to complex formation between Ag and the organic molecules. The best method of preparation of perbromate ion is by reaction 1.18. Cation exchange can be used to give HBrO4, but the anhydrous acid has not been isolated.
 (1.18)
(1.18)
Potassium perbromate has been structurally characterized and contains tetrahedral [BrO4]- ions (Br_O = 161 pm). Thermochemical data show that [BrO4]- (half-reaction 1.19) is a slightly stronger oxidizing agent than [ClO4]- or [IO4]- under the same conditions. However, oxidations by [BrO4]- (as for [ClO4]-) are slow in dilute neutral solution, but more rapid at higher acidities.
 (1.19)
(1.19)
Several different periodic acids and periodates are known; Table 1.1 lists periodic acid, HIO4 and orthoperiodic acid, H5IO6 (compare with H6TeO6). Oxidation of KIO3 by hot alkaline hypochlorite yields K2H3IO6 which is converted to KIO4 by nitric acid; treatment with concentrated alkali yields K4H2I2O10, and dehydration of this at 353K leads to K4I2O9. Apart from [IO4]- (1.3) and [IO5]3- and [HIO5]2- (which are square-based pyramidal), periodic acids and periodate ions feature octahedral I centres, e.g. H5IO6 (1.4), [H2I2O10]4- (1.5) and [I2O9]4- (1.6).
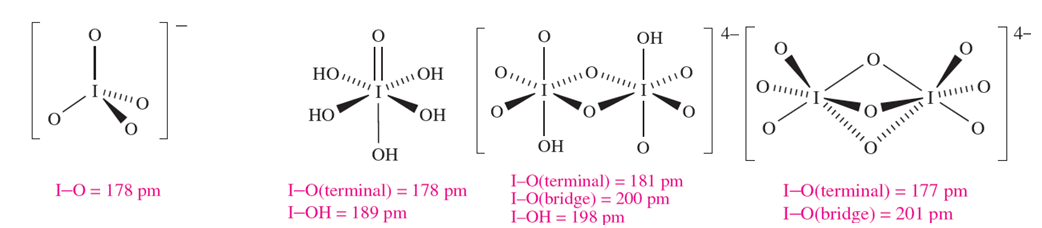
(1.3) (1.4) (1.5) (1.6)
The relationships between these ions may be expressed by equilibria 1.20, and aqueous solutions of periodates are therefore not simple systems.
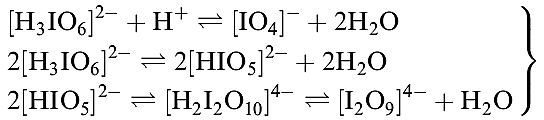 (1.20)
(1.20)
Orthoperiodic acid is obtained by electrolytic oxidation of iodic acid, or by adding concentrated nitric acid to Ba5(IO6)2, prepared by reaction 1.21.
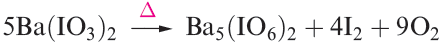 (1.21)
(1.21)
Heating H6IO6 dehydrates it, first to H4I2O9, and then to HIO4. In aqueous solution, both H6IO6 (pKa = 3.3) and HIO4 (pKa= 1.64) behave as rather weak acids. Periodate oxidizes iodide (equation 1.22) rapidly even in neutral solution (compare the actions of chlorate and bromate); itliberates ozonized O2 from hot acidic solution, and oxidizes Mn(II) to [MnO4]-.
 (1.22)
(1.22)
 الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















