

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Group 1: alkali metal organometallics
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المصدر:
INORGANIC CHEMISTRY
الجزء والصفحة:
2th ed p 55
28-2-2017
2782
Group 1: alkali metal organometallics
Organic compounds such as terminal alkynes (RC≡CH) which contain relatively acidic hydrogen atoms form salts with the alkali metals, e.g. reactions 1.1and 1.2 .
 (1.1)
(1.1)
 (1.2)
(1.2)
Similarly, in reaction 1.3, the acidic CH2 group in cyclopentadiene can be deprotonated to prepare the cyclopentadienyl ligand which is synthetically important in organometallic chemistry Na[Cp] can also be made by direct reaction of Na with C5H6. Na[Cp] is pyrophoric in air, but its air-sensitivity can be lessened by complexing the Na ion with 1,2-dimethoxyethane (dme). In the solid state, [Na(dme)][Cp] is polymeric (Figure 1.1).
 (1.3)
(1.3)

Fig. 1.1 Part of a chain that makes up the polymeric structure of [Na(dme)][Cp] (dme=1,2-dimethoxyethane); the zig-zag chain is emphasized by the hashed, red line. The structure was determined by X-ray diffraction [M.L. Coles et al. (2002) J. Chem. Soc., Dalton Trans., p. 896]. Hydrogen atoms have been omitted for clarity; colour code: Na, purple; O, red; C, grey.
A pyrophoric material is one that burns spontaneously when exposed to air. Colourless alkyl derivatives of Na and K are obtained by transmetallation reactions starting from mercury dialkyls (equation 1.4).
 (1.4)
(1.4)
Organolithium compounds are of particular importance among the group 1 organometallics. They may be synthesized by treating an organic halide, RX, with Li (equation 1.5) or by metallation reactions (equation 1.6) using nbutyllithium which is commercially available as solutions in hydrocarbon (e.g. hexane) solvents.
 (1.5)
(1.5)
 (1.6)
(1.6)
Solvent choices for reactions involving organometallics of the alkali metals are critical. For example, nBuLi is decomposed by Et2O to give nBuH, C2H4 and LiOEt. Alkali metal organometallics are extremely reactive and must be handled in air- and moisture-free environments; NaMe, for example, burns explosively in air.
Lithium alkyls and aryls are more stable thermally than the corresponding compounds of the heavier group 1 metals (though they ignite spontaneously in air) and mostly differ from them in being soluble in hydrocarbons and other nonpolar organic solvents and in being liquids or solids of low melting points. Sodium and potassium alkyls are insoluble in most organic solvents and, when stable enough with respect to thermal decomposition, have fairly high melting points. In the corresponding benzyl and triphenylmethyl compounds, Na+[PhCH2]- and Na+[Ph3C]- (equation 1.7), the negative charge in the organic anions can be delocalized over the aromatic systems; this enhances stability and the salts are red in colour.
 (1.7)
(1.7)
Sodium and potassium also form intensely coloured salts with many aromatic compounds (e.g. reaction 1.8). In reactions such as this, the oxidation of the alkali metal involves the transfer of one electron to the aromatic system producing a paramagnetic radical anion.
 (1.8)
(1.8)
Table 1.1 Degree of aggregation of selected lithium alkyls at room temperature (unless otherwise stated).
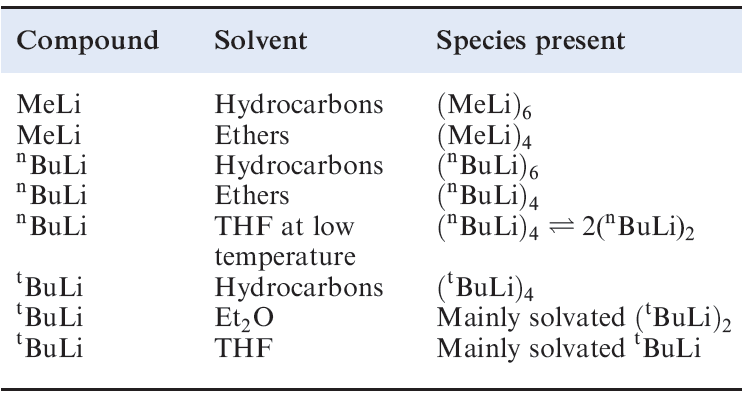
A radical anion is an anion that possesses an unpaired electron. Lithium alkyls are polymeric both in solution and in the solid state. Table 1.1 illustrates the extent to which MeLi, nBuLi and tBuLi aggregate in solution. In an (RLi)4 tetramer, the Li atoms form a tetrahedral unit, while in an (RLi)6 hexamer, the Li atoms define an octahedron. Figures 1.2a and b show the structure of (MeLi)4; the average Li_Li bond length is 261 pm compared with 267pm in Li2 , the bonding in lithium alkyls is the subject of problem 1.2 at the end of the chapter. Figures 1.2c and d show the structure of the Li6C6-core of (LiC6H11)6 (C6H11 = cyclohexyl); six Li_Li bond distances lie in the range 295–298pm, while the other six are significantly shorter (238–241pm). The presence of such aggregates in solution can be determined by using multinuclear NMR spectroscopy. Lithium possesses two spin-active isotopes and the solution structures of lithium alkyls can be studied using 6Li, 7Li and 13C NMR spectroscopies as worked example 1.1 illustrates. The alkyls of Na, K, Rb and Cs crystallize with extended structures or are amorphous solids.
 الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















